Source: JPRI
Abstract
Coronavirus disease 2019 (COVID-19) is emerging contagious pneumonia due to the new Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). It initially appeared in Wuhan China in December 2019 then rapidly spread worldwide and became a pandemic. For the time being, there is no specific therapeutic treatment for this disease.
Herein, the “state-of-the-art” of treatment modalities was systematically reviewed and ultimately a practical therapeutic algorithm for the COVID-19 management was proposed. The systematic review was performed by using published articles retrieved from Science Direct, MEDLINE, and Scopus databases concerning this topic. Among 1060 articles collected from the different databases, 19 publications were studied in-depth and incorporated in this review.
The most three frequently used medications for the treatment of COVID-19 was: the available anti-viral drugs (n= 9), the antimalarial hydroxychloroquine or chloroquine (n = 8), and the passive antibody transfer therapy (n = 2). Among all treatment modalities, antimalarial hydroxychloroquine ranked the highest cure rate.
Therefore, this drug is considered as the first‐line of COVID-19 treatment. The second‐line treatment includes the lopinavir/ritonavir drugs combined with interferon β-1b and ribavirin. Finally, the third‐line treatments include the remdesivir drug and passive antibody transfer therapy. However, our review emphasis the urgent need for adequately designed randomized controlled trials, enabling a more significant comparison between the most used treatment modalities.
1. INTRODUCTION
A new human coronavirus causing acute respiratory disease has emergedin Wuhan, China since December 2019. This new virus and the resulted illness were termed as the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the Coronavirus disease 2019 (COVID-19), respectively. The human to human transmission mode ofSARS-CoV-2 virus has led to pandemic. As of August 1st 2020, more than 18 million of COVID-19 cases have been reported in the World including 688, 289deaths [1].
The human to human transmission occurs through close contact with patients during coughing, sneezing, or discussing without any protective measures.The clinical manifestation of the disease varies from mild symptoms (non-pneumonia), to mild to moderate symptoms (mild pneumonia) and severe disease (dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation (SpO2) ≤ 93%, PaO2/FiO2 ratio < 300, and/or lung infiltrates > 50% within 24 to 48 hours).
The extremely rapid pandemic worldwide associated with the absence of specific treatment and vaccine has resulted in a severe public health risk. Thereby, in February 2020, the World Health Organization (WHO) raised the threat of the COVID-19 epidemic to the “very high” level. Since that time, scientists all over the globe have started working on different aspects related to SARS-CoV-2 including the clinical features and consequences of the disease, the transmission routes, emerging diagnostic approaches as well as prevention and therapeutic strategies.
Thereby, getting treatment for and prevention against SARS-CoV-2 infection became an urgent need for policy-makers and public health professionals to stop the pandemic through: i) establishing proper guidelines for the treatment of the COVID-19 suspected/confirmed patients using previously known drugs, and ii) developing vaccines neutralizing SARS-CoV-2 virulence antigens.Hundreds of clinical trials are being conducted worldwide, striving to treat COVID-19. They tested HIV antiretroviral drugs and LPV/RTV to treat patients infected with SARS-CoV-2 [2,3].
Other physicians assessed the effect of other commonly used antiviral drugs such as Remdesivir, Umifenovir and combination of 2 anti-viral molecules or an anti-viral drug with interferon on SARS-CoV-2 proliferation [4–9]. Passive antibody transfer using convalescent’s plasma therapy approach as well as polyclonal immunoglobulin were also invistigated [10,11]. All data derived from these critical preliminary investigations did not definitively confirm the effectiveness of these different medications for the treatment of or prevention against the SARS-CoV-2 infection.
In the current context of the COVID-19 pandemic, with the absence of a therapeutic consensus, our review aimed to discuss the outcomes of the recently tested therapeutic modalities. Such an updated systematic review should point out the effectiveness, reliability, adverse events of each alternative, thereby learning lessons from these clinical trials and provides evidence-based recommendations for future therapeutic essays.
2. MATERIALS AND METHODS
This systematic review was conducted in consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews [12,13]. The systematic review process was carried out as follows: i) literature search, ii) data extraction and analysis and iii) description of the results and translation into practicalguidelines.For this, three clinical topics related to COVID-19 were formulated and used to direct the sub-topics of the systematic review.
This includes:
i) the treatment with anti-malarial drugs (key words: Chloroquine, hydroxychloroquine and COVID-19),
ii) the treatment with anti-viral drugs (Keys words: anti-viral drugs and COVID-19) and iii) the immunotherapy treatment (key words: Immunotherapy and COVID-19).
Thus, collected articles during the systematic review were categorized into these three topics.The systematic search was conducted by searching in MEDLINE (PubMed), ScienceDirect and Scopus databases to identify relevant articles from January 2000 to May 23th2020 using keywords according to the three topics mentioned above. Unpublished clinical studies were excluded from this study. All eligible collected articles were preliminarily nominated based on the title and content of the abstract of each article.
Selected articles were identified and data extraction concerning the study (authors, date of publication, type of study and sample size), the therapeutic modality (anti-coronavirus molecule, dosage and dose, duration and control group) and the main findings (clinical outcomes, viral clearance, adverse events and mortality) were performed. Results obtained from the selected studies underwent a descriptive summary of the statement of evidence. According to the results obtained from all articles, a proposed therapeutic algorithm for COVID-19 was structured.
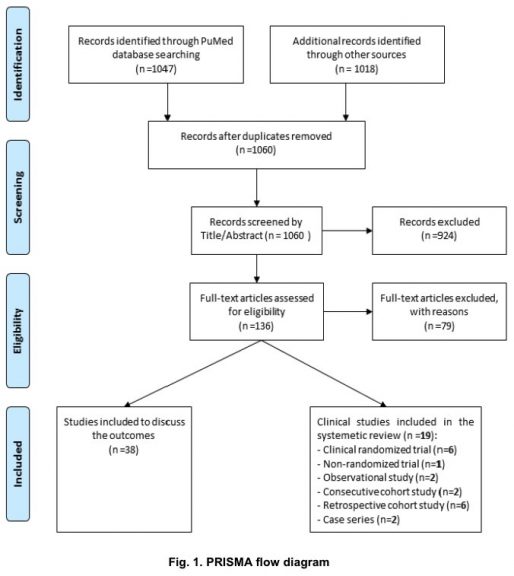
A flow chart describing the selection criteria used in the systematic review. A pool of 1060 articles was extracted from different research databases as indicated. Irrelevant or less relevant articles (case studies, short communications, articles with unwell described methodology) were then excluded based on title and abstract (n=136). Eligible articles were identified after concise full text reading (n=57). Nineteen out of fifty seven articles were used as the basis of the review while thirty nine were considered in the discussion.3.
RESULTS
We retrieved 1060 articles from the cited databases. Among them, 924 were excluded after careful checking of titles and abstracts. The rest of the articles (136) were further checked for eligibility and non-eligible articles were excluded (n=79).
Ultimately, 57 eligible studies were split into two groups including 19 studies that have been used as the backbone of the review, while 38 studies were used as supporting studies for further discussion. The 19 legible studies presented the results of randomized clinical trials (n=6), non-randomized trial (n=1), observational study (n=2), consecutive cohort study (n=2), retrospective cohort study (n=6) or case series (n=2), as shown in Fig. 1. These studies have been conducted in China (n=12), France (n=3), Italy (n=1), Republic of Korea (n=1), Brazil (n=1), and USA (n=1).
The studies included patients with mild COVID-19 symptoms (n=8 studies), mild to moderate COVID-19 symptoms (n=3 studies), moderate and severe COVID-19 symptoms (n=2 studies) and severe COVID-19 symptoms (n=5 studies). Data concerning the clinical status of COVID-19 patients were missing from one study.
The interventions concerned CQ(n=2) [14,15], HCQ(n=6) [16–21], remdesivir (n=2) [4,5] LPV/RTV (n=4) [2,3,6,22], Umifenovir or Arbidol (n=2) [7,9], the combination of Arbidol with interferon IFN-α2b (n=1) [8], the injection of high dose of immunoglobulin (n=1) [10] and the transfusion of convalescent plasma (n=1) [11]. A comprehensive summarie of the outcomes of the literatures reviewed in this study is shown in Table1. It includes description of the studies, the treatment molecules used and their dose and duration, major findings, adverse effects and the mortality rate.
3.1 Chloroquine and Hydroxychloroquine
CQ and HCQ are two 4-aminoquinoline drugs that have inhibitory effects on pro-inflammatory cytokine production, DNA replication and chemotaxis [23]. They have been widely used in the treatment of malaria, amoebiasis and some autoimmune diseases [24]. Moreover, they have earned a reputation for the inhibition and spread of many viruses such as HIV and SARS-CoV-1 [25,26]. It is known that HCQ is a metabolite of CQ and both of them have the same mechanism of action. They are weak bases that increase the pH of acidic vesicles like endosomes, Golgi vesicles and lysosomes. They have been additionally proved to decrease the affinity of angiotensin-converting enzyme-2 (ACE2) 1 by impairing terminal glycosylation of ACE2 and decrease viral infectivity [25]. As a result of the occurrence of the COVID-19 pandemic, many research teams have directly recruited these molecules and tested their impact on the SARS-CoVin vitrousing cultured cells as an emodel. Wang and colleagues have demonstrated the efficiency of CQ in inhibiting the replication of SARS-CoV-2 at an EC50 of 1.13 μM in cells. It has been speculated that, as previously shown for other viral infections, QC might alter the virion assembly by interfering with the maturation of the M protein failing phagosome formation [25,27,28].




An extensive study by the same group revealed that HCQ is more efficient in inhibiting SARS-CoV-2 infection and these molecules can alter viral entry into the target cells. Additionally, the study revealed that the transport of the virion within the host cell is affected due to the blocking of the endosome maturation at intermediate stages of endocytosis, leading to frustration of virion trafficking to the ultimate releasing site [29].
Furthermore, HCQ has been reported to be less toxic with higher efficiency in preventing SARS-CoV-2 infection than CQ in vitro[29,30]. Such promising experimental studies-derived findings have encouraged clinicians to use these two molecules for the treatment of COVID-19 confirmed cases. Thus, as of May 23th, 2020 a total of eight clinical studies including two randomized clinical trials, two retrospective studies, two observational studies, one non-randomized clinical trial and one consecutive cohort study were published [14–21].
These studies have resulted in sometimes contradictory findings. A total of 181 and 1412 patients were treated with CQ and HCQ-respectively. A Brazilian randomized clinical trial using 2 different dosages that includes both a low dose (450 mg, 2 doses on the first day followed by a single-daily dose on the next 4 days) and a high dose (600 mg, , in double-daily dose for 10 days) of CQ diphosphate was conducted on 81 COVID-19 patients.
This trial has shown that there was no clear benefit of CQ regarding the lethality rate among patients. Therefore, the authors have recommended avoiding the use of high dosage of CQ in particular for those with previous cardiac diseases [15]. However, a Chinese clinical study on more than 100 patients treated has demonstrated that CQ (i) inhibits the exacerbation of pneumonia, (ii) improves lung imaging finding, (iii) promotes the virus-negative conversion and (iv)accelerates the disease recovery [14].
Concerning the HCQ treatment regimen, a Chinese randomized controlled trial was carried on 148 individuals experiencing with mild to moderate and 2 additional cases with severe symptoms of disease, respectively. Among this group 75 patients were treated with HCQ (1200 mg/day for three days followed by 800 mg/day along 14 days for patients with mild to moderate illnesses and 21 days for those with severe illness) and 75 patients were treated with the standard care regimen only.
This trial has shown that there is no significant difference in the viral clearance between the two groups [20]. Gautret et al. 2020 have conducted two studies, one non-randomized clinical trial and one pilot observational study, assessing the effectiveness of the combination of HCQ with Azithromycin (HCQ-AZ) for the administration of confirmed COVID-19 cases with mild symptoms. A total of 100 patients has benefited from this treatment regimen (HCQ: 200 mg in a triple-daily dose for 10 days and AZ: 500 mg on day 1 followed by 250 mg in a single-daily dose for 4 days).
These two French clinical studies have proved that HCQ-AZ led to a significant of nasopharyngeal viral load.Interestingly, it has been demonstrated that AZ reinforced the HCQ effect with only mild adverse events [16,17]. A consecutive study conducted in USA has assessed the impact of HCQ-AZ on the prolongation of QT-interval (the time of ventricular activity including both depolarization and repolarization) among 84 patients.
The study revealed prolongation of the corrected QT-interval from a baseline average of 435 ± 24 ms to a maximal average value of 463 ± 32 ms (P<0.001), suggesting that a repeated follow up of this parameter during the treatment period should be taken in concern [18]. A French observational comparative study conducted on 181 patients with COVID-19 pneumonia requiring oxygen demonstrated that treatment of COVID-19 patients (n=84) with 600 mg/day of HCQ for a period of 10 days has no positive impact on reducing the needs of transmitting the patients to intensive care unit (ICU) and lethality rate [19].
Finally, the latest retrospective 1061 infected persons with mild COVID-19 in French hospitals showed that the combination HCQ-AZ (HCQ: 200 mg in a triple-daily dose for 10 days and AZ: 500 mg on the first day followed by 250 mg/day for 4 days) resulted in a significantly efficient recovery of 91.7% patients, with a significant decreasein fatality rate [21].
Collectively, a daily of 3 doses of 200 mg for 10 days combined with 500 mg AZ on first day followed by 250 mg/day for the next four days results in a highly successful treatment of COVID-19 patients with mild adverse events (such as diarrhea, vomiting, nausea, abdominal discomfort and headache). Nevertheless, CQ and HCQ should not be prescribed for patients with contraindications including patients less than 14 years old, allergy/intolerance to CQ, HCQ or AZ, cardiac pathology (prolonged corrected QT, Brugada syndrome, myocarditis history) ophthalmologic pathologies (retinopathy, glaucoma, accommodation disorder), G6PD deficiency, epilepsy and hypokalemia [21].
3.2 Remdesivir
Remdesivir (GS-5734) is an analogue of adenosine triphosphate and a phosphoramidate prodrug with a broad spectrum activity against several virus families including Coronaviridae(Such SARS-CoV-1 and Middle East respiratory syndrome coronavirus [MERS-CoV]) [31,32]. It is used as a substrate for many viral RNA-dependent RNA polymerase (RdRp) complexes.
It affects the nascent viral RNA chains resulting in premature chain termination of viral RNA transcription [33]. It was already demonstrated that remdesivir possesses an in vitro and in vivo activity against all the animals and human coronaviruses including SARS-CoV-1 and MERS-CoV [34–37].
Subsequently, and since the COVID-19 outbreak, many researchers have renewed their interest in remdesivir for the elimination of SARS-CoV-2. Thus, in vitro infection essay has shown a potent antiviral activity of Remdesivir [27]. The first reported case treated with remdesivir was by Holshue et al, 2020 in USA. This patient recovered significantly after one day of receiving the first dose of remdesivir without more requirement of oxygen [38].
Another report included 53 severe and critical cases who showed clinical improvement (68%) after treatment with a course of remdesivir for 1-10 days [39]. Though, the small size of the treated patients, the lack of data on some patients as well as the absence of a control group prevented clinicians to draw solid conclusions from such studies.Up to date, one randomized double-blind, placebo-controlled multi-centre trial and one prospective open-label study were published concerning the use of remdesiviras a therapeutic regimen for patients with severe symptoms of COVID-19 [4,5].
The first randomized trial was conducted in China, including ten hospitals in Hubei. A total of 158 patients have benefited from intravenous remdesivir (200 mg on the first day followed by a daily infusion of 100 mg along the next 9 days) versus 79 patients have received identical volumes of placebo infusions along the same period. Unfortunately, no significant clinical benefit was retrieved from this trial.
Additionally, no significant difference neither in mortality (14% death in remdesivir-recipients group vs. 13% in placebo-recipients group) nor in viral load was achieved between the two groups. Nevertheless, patients receiving remdesivir showed a faster recovery time in comparison to those receiving placebo. Ultimately, adverse events were reported in 66% and 64% of remdesivir and placebo recipients respectively [4].
The latest prospective open-label study (published on May 2020) was performed at Luigi Sacco Hospital, Italy and has included 35 patients from which 18 were admitted in the ICU while 17 patients were hospitalized outside ICU. On day 28, 82.3% of patients outside ICU were discharged versus 33.3% of the ICU group. Besides, 44.4% of ICU patients died [5]. The authors concluded that Remdesivir could have a beneficial effect on COVID-19 in non-critically ill patients.Accordingly, till now, compassionate use of remdesivir was restricted for patients with severe and ICU-hospitalized patients. Currently, no evidence-based conclusion could be drawn for mild to moderate COVID-19 patients.
3.3 Lopinavir/Ritonavir
LPV is a protease inhibitor that is used in parallel with RTV as a treatment regimen for patients with Acquired immunodeficiency syndrome (AIDS). It targets theHIV-1 protease resulting in immature non-infectious virions [40]. RTV has no direct effect on viruses but extends bioavailability of LPV by blocking the host’s cytochrome P450 3A4 enzyme and thereby boosting LPV concentration [41].
Previously, in vitro experimental studies have reported the inhibition effect of LPV on the replication of SARS-CoV-1 and MERS-CoV with EC50 at 17.1 μM and 8 μM, respectively [42,43]. The same drug has demonstrated high recovery outcomes when it was used in the administration of non-human primate models(common marmosets) suffering from a severe disease mimicking the human MERS [44].
Recently, Choy et al. have assessed the impact of Remdesivir, LPV, emetine, and homoharringtonine against SARS-CoV-2 in vitro. In this study, the authors demonstrated that LPV has an antiviral effect on SARS-CoV-2 with EC50 at 26.1 μM [45]. Another study reported the improvement of clinical symptoms and the rapid and drastic decrease of viral load in a patient with mild symptoms treated with LPV/RTV [46].
As of May 23th, 2020, three randomized controlled trials and two retrospective cohort studies were published and had assessed the effectiveness and safety of LPV/RTV monotherapy or in combination with other molecules as a possible improved treatment of COVID-19 [1,2,6,7,22]. All randomized controlled trials were conducted in China. They have involved a total of 412 patients, of whom 213 and 199 suffered from mild to moderate and severe
illnesses, respectively [2,3,22].Out of this population, 117 patients were included as a control group receiving a standard care regimen. LPV/RTV monotherapy has shown little benefit regarding the improvement of the clinical outcomes of hospitalized COVID-19 patients with the same mortality rate among the treated and control groups [2,3].
However, the most recent open-label randomized phase 2 trial performed in China has evaluated the combination LPV/RTV, ribavirin and interferon β-1b (ABT-378 /RTV: 400 mg/100 mg twice a day, ribavirin 400 twice a day, and 3 doses of 8 million IU of interferon β-1b for 11 days) for the treatment of patients with mild to moderate symptoms of the disease.
This study demonstrated that triple antiviral therapy result in alleviating symptoms and shorten the time period of viral clearance compared to the control group who received a LPV/RTV monotherapy regimen [22]. Another study included 16cases with mild symptoms of the disease that were treated with the combination of LPV/RTV/Arbidol versus 17 patients treated by LPV/RTV only, have highlighted the benefit of the combination in improving the clinical outcomes of patients. Indeed, 14 days post-treatment, the virus was not detected in 94% and 52.9% of patients treated with LPV/RTV/Arbidol and LPV/RTV only, respectively [6].
A similar retrospective study comparing the clinical and viral outcomes of two groups of patients (one treated with LPV/RTV and one treated with Arbidol only) has reported no significant differences in fever duration between the two groups [7]. After 14 days, no viral load was detected in the Arbidol group, while it was detected in 44.1% patients treated with LPV/RTV, implying that Arbidol monotherapy seems to be more efficient than LPV/RTVin the treatment of COVID-19. However, no definitive conclusion could be decided since these results were not retrieved from a randomized controlled trial.
Accordingly, LPV/RTV monotherapy has negative to weak efficacy for the treatment of COVID-19 patients. Its association with interferon β-1b and ribavirin has proved a superior effect in alleviating symptoms and viral clearance. LPV/RTV(400/100 mg every 12 hours) combined with ribavirin (400 mg every 12 h) and interferon beta-1b (3 doses of 8 million IU on alternate days) for 14 days can be used as the second choice if HCQ/CQ was contraindicated. For optimal effectiveness, it was suggested to administer this antiviral therapy within 10 days after symptoms onset[47].3.4 Umifenovir (Arbidol) Umifenovir is an antiviral drug used for the treatment of influenza A and B virus infections in Russia and China. The action mode of this molecule is direct by the inhibition of virus proliferation and indirect through the induction of interferon and immune cell production [48,49].
In 2008, Khamitov et al. assessed the action of Arbidol on SARS-CoV-1 virus using cultured cells and reported a promising antiviral activity of this molecule [50]. By the outbreak of COVID-19, the Chinese National Health Commission published the guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection. It recommended the combination of IFN-α-Arbidol as a therapeutic option [51].
However, up-to-date, clinical studies on the Arbidol effect on COVID-19 patients are scarce. By making a thorough literature review, we find only three published retrospective cohort studies (total of 236 patients) two of them have assessed the effect of Arbidol monotherapy on COVID-19 patients [7,9]and a single study has evaluated the combination Arbidol/Interferon (IFN)-α2b [8]. Overall, Arbidol monotherapy did not prove any benefit for enhancing the clinical and viral outcomes of patients with mild, moderate or severe COVID-19 compared to the control group (with standard care regimen only) [9]. Indeed, after one week of treatment (0.2 g Umifenovir in triple-daily doses for 5-10 days), no positive effect was obtained.
In contrast, the study team observed higher median time for hospitalization of the Umifenovir-treated patients in comparison to the control patients. Another retrospective Cohort Study included 70 patients treated with IFN-α2b only and 71 patients treated with the combination of Arbidol/IFN-α2b.
This study reported that this last combination had no benefit for SARS-CoV-2 RNA clearance and hospitalization compared to IFN-α2b monotherapy. Besides, the clearance duration of the viral RNA in the IFN-α2b monotherapy group was not longer than that obtained in the combined therapy group [8]. Finally, the authors concluded that Arbidol/IFN-2b therapy could positively enhance the SARS-Cov-2-induced pneumonia of mild patients, but it remains unable to accelerate the virus clearance.
3.5 Passive Antibodies Transfer
Another promising treatment regimen option for COVID-19 patients is the passive transfer of antibodies. This therapeutic alternative gathers two strategies: (i) the administration of convalescent plasma containing neutralizing antibodies or (ii) the administration of high-dose intravenous immunoglobulin (Ig) containing polyclonal IgG antibodies.
The first strategy is known as an empirical therapeutic option previously used for the treatment of SARS-CoV-1, H1N1 influenza, Ebola and MERS [52–54]. It consists of plasma collected from individuals with a prior confirmed diagnosis of the relative infection and who have recovered at least 14 days prior to donation with negative PCR evidence for the presence of the intended virus.
This strategy has demonstrated an interesting benefit in enhancing clinical outcomes, shortening hospital stay, dropping the respiratory tract viral load and reducing the mortality rate related to these infections [52-54]. These previous promising findings have encouraged clinicians to try the transfusion of convalescent plasma to the SARS-CoV-2 severely-infected individuals. Thus, a preliminary finding of a case series, including five severe cases of SARS-Cov-2 induced pneumonia and benefiting from the transfer of convalescent plasma was recently published [11].
The administration of convalescent plasma with a SARS-CoV-2 IgG titer higher than 1:1000 by ELISA and neutralizing antibody titer >40 resulted in (i) the normalization of body temperature after 3 days (ii) the decrease of the sequential organ failure assessment score and the negativity of viral load after 12 days and (iii) the resolving of the acute respiratory distress syndrome [11].The second strategy for passive antibodies transfer is the administration of High-Dose Intravenous Immunoglobulin (IVIg).
It is a blood product isolated from healthy donors containing polyclonal IgG and bioactive moieties and has an immunomodulatory effect [55]. It was previously used for the treatment of SARS and MERS and demonstrated many clinical benefits and good tolerance [56,57]. A preliminary study of a series of three patients with severe COVID-19 that reported that the administration of High-Dose Intravenous Immunoglobulin was effective to improve the clinical outcomes and viral overcome in these patients. Indeed, it led to a progressive enhancement of clinical status, partial resolution of lung lesions and negative detection of virus from oropharyngeal swab [10].

Results obtained from the selected studies were summarized accordingly. Specification of proposed treatment strategy and the corresponding drug concentration/plasma titer for sever, moderate and mild illnesses are : Hydroxychloroquine, AZ: Azithromycin, LPV/RTV/RBV: lopinavir/ritonavir/ribavirin
Although these findings are promising, the small number of COVID-19 patients recruited in this treatment strategy prevents clinicians from concluding its effectiveness. For this, controlled randomized trials are urgently needed to evaluate the treatment by passive antibodies transfer accurately.
4.CONCLUSION AND PROPOSED ALGORITHM
Based on the analysis of the published clinical studies concerning the treatment of COVID-19 patients and with the absence of a specific treatment and vaccine for SARS-CoV-2, clinicians are still obliged to deal with the available treatment strategies with a lot of precautions.
Thus, in the absence of any contraindication, a combination of HCQ-AZ can result in good clinical and infection recovery outcomes for patients with mild to moderate illness. Nevertheless, this treatment regimen should be closely gathered with a follow up of vital parameters and the electrocardiogram of the patient. In the case of any contraindications, this treatment can be substituted by LPV/RTV combined with interferon β-1b and ribavirin to alleviate symptoms and eliminate the virus. Another alternative is the treatment with the combination of Arbidol/IFN α-2b. Compassionate use of remdesivir should be prescribed for patients with severe COVID-19 and hospitalized in ICU.
In case of the availability of eligible donors, convalescent plasma is considered as a promising alternative treatment for critically ill patients (Fig. 2).This evidence-based algorithm derived from the analysis of clinical studies may help clinicians to manage the COVID-19 cases in their respective hospitals and point out the property of each treatment strategy of choice on efficacy and safety outcomes.
Nevertheless, it is still primordial to conduct more large-scale clinical trials to assess the efficiency of the available treatment strategies accurately.
CONSENT
It is not applicable.
ETHICAL APPROVAL
It is not applicable.
ACKNOWLEDGEMENT
This research has been funded by the Scientific Research Deanship at University of Ha’il -Saudi Arabia through project number COVID-1938.
COMPETING INTERESTS
Authors have declared that no competing interests exist
Related:
Belgium Study 8,075 patients: Low HCQ doses resulted in lower mortality in Covid patients
Risk of Death Is 30% Lower for COVID-19 Patients Treated With Hydroxychloroquine

