Source: Science Direct, the Full PDF is HERE
1 INTRODUCTION
2 Hydroxychloroquine (HCQ) has shown efficacy against COVID-19 in some but not all studies. We
3 hypothesized that systematic review would show HCQ to be: effective against COVID-19, more effective
4 when used earlier, not associated with worsening, and safe.
5 METHODS
6 We searched PubMed, Cochrane, EmBase, Google Scholar, and Google for all reports on
7 hydroxychloroquine as a treatment for COVID-19 patients. This included pre-prints and preliminary
8 reports on larger COVID-19 studies. We examined the studies for efficacy, time of administration and
9 safety.
10 RESULTS
11 HCQ was found consistently effective against COVID-19 when used early, in the outpatient setting. It
12 was found overall effective also including inpatient studies. No unbiased study found worse outcomes
13 with HCQ use. No mortality or serious safety adverse event was found
14 CONCLUSIONS
15 HCQ is consistently effective against COVID-19 when used early in the outpatient setting, it is overall
16 effective against COVID-19, it has not produced worsening, it is safe.
17
18
19
20
21
22
23
24 Journal Pre-proof
25 Introduction
26 There is a need for effective treatment for COVID-19 infection. Hydroxychloroquine (HCQ), with or
27 without azithromycin, has been found to have efficacy as a treatment for COVID-19 in some studies [1,
28 2], while other studies have not shown efficacy[3, 4]. While we do not prescribe HCQ to typical patients,
29 we do treat various forms of inflammatory arthritis in patients taking HCQ prescribed from outside
30 providers. Some physicians have stated that HCQ has greater efficacy if given earlier in the course of the
31 disease[5, 6]. Several studies showing negative efficacy have been withdrawn due to methodological
32 improprieties [7]. We hypothesized that HCQ clinical studies would show significant efficacy more often
33 than not for COVID-19; and that efficacy would be greater if HCQ were used earlier in the course of the
34 disease. We also hypothesized that some studies that failed to show efficacy would be biased against
35 positive efficacy and that no unbiased studies would show worsening. We also hypothesized that HCQ
36 would be found to be safe.
37 Methods
38 We searched PubMed, Cochrane, EmBase, Google Scholar, and Google for all reports on
39 hydroxychloroquine as a treatment for COVID-19 patients. This included pre-prints and preliminary
40 reports on larger COVID-19 studies. We included papers with HCQ alone as well as in combination with
41 Azithromycin and/or Zinc. We excluded papers that studied Chloroquine. While Chloroquine has
42 shown efficacy it has a higher side effects profile than HCQ. For this reason, and because HCQ is
43 inexpensive and widely available we believe that future treatment will and should focus on HCQ. It was
44 thus our priority to examine HCQ as fully as possible. We excluded papers that only examined
45 hydroxychloroquine as a means to decrease transmission of coronavirus since our focus was on
46 demonstrated clinical efficacy. Reports were analyzed for efficacy, type of study, time of intervention
47 with HCQ during the COVID-19 disease course, and for adverse events. Our final search was performed
August 3rd 48 , 2020.
49 Results
50 A total of 43 reports were found that examined hydroxychloroquine treatment for COVID-19 patients.
51 25 found positive clinical efficacy from using hydroxychloroquine for COVID-19 patients; 15 showed no
52 improvement with HCQ, and 3 showed worse clinical results in patients who received HCQ.
53 11 of the studies found in our review examined HCQ efficacy on patients in the outpatient or “day
54 hospital” setting and all reported positive results [8]. However in two of the studies [9, 10] the positive
55 results, while clinically important (decreased risk of hospitalization and improvement in symptom
56 resolution), were not statistically significant.
57 We found 32 reports of HCQ treatment in hospitalized patients with COVID-19. Of these 32 reports of
58 hospitalized patients, 14 reported good results, 15 reported no improvement and 3 reported worse
59 results. 14 studies reported the time during treatment at which HCQ was initiated. In nine studies HCQ
60 was administered within 48 hours of admission. In six [11-16] of these nine, improvement was noted. In
61 three no improvement was noted [3, 17, 18]. In five studies HCQ was administered more than 48 hours
62 after admission or in the ICU. In two [19, 20] of these five improvement was noted. In three it was not
63 [21-23]. In 18 studies the time of administration was not specified.
64 Seven of the 43 total studies [12, 17, 20, 24-27] were chartless retrospective studies that used only
65 billing codes. These studies all allowed initiation of HCQ treatment at times that differed with initiation
66 of the control treatment: with HCQ presumably being chosen at the physician’s discretion in worsening
67 patients more in need of treatment. All such studies were felt to exhibit selection bias against a positive
68 result. Four additional studies [9, 10, 15, 16] had positive trends toward efficacy that did not reach
69 statistical significance. In 1 study [22] 8% of the treatment group was untreated but not excluded from
70 the treatment group calculations. In addition the median level of treatment was only 67% of the
71 specified treatment. 19 of the 43 papers were pre-prints or otherwise not peer reviewed. 24 of the
72 papers were from peer reviewed journals. Of the eleven outpatient papers, all of which showed positive
73 results, 7 were peer reviewed, 4 were not. Of the 32 hospitalization papers 17 were peer reviewed and
74 15 were not. Overall 12 of 24 or 50% of the peer reviewed papers, and 11 of 19 or 58% of the non-peer
75 reviewed papers showed positive efficacy.
76 Some studies used HCQ alone, some had the addition of azithromycin or zinc. No outcome difference
77 was seen with the addition of azithromycin (table 4), although all of the outpatient studies that used
78 Azithramycin had a positive result. There were no deaths reported as a result of HCQ, azithromycin or
79 Zinc treatment. Increased QTc was seen but not Torsades de Pointes. Adverse events that were felt to
80 be likely due to HCQ treatment were non-life threatening. No permanent sequelae were described.
81 Adverse events are listed in Tables 1-3.
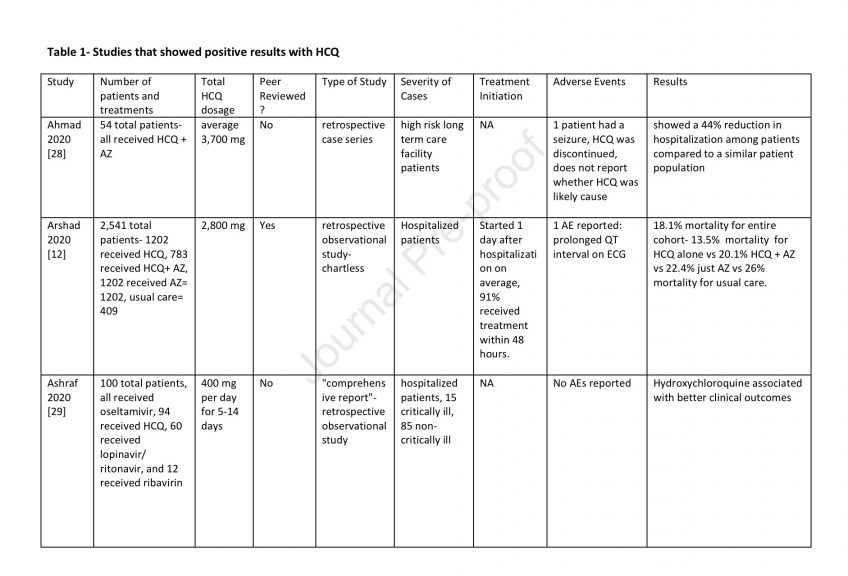













82 Discussion
83 This study has four important findings. The first is that HCQ appears to be consistently effective for the
84 treatment of COVID-19 when used early in the course of disease in the outpatient setting, and is
85 generally more effective the earlier it is used. The second is that overall HCQ has had efficacy against
86 COVID-19 in a majority of studies. The third is that there are no unbiased studies showing a negative
87 effect of HCQ treatment of COVID-19. The fourth is that HCQ appears to be safe for the treatment of
88 COVID-19 when used responsibly.
89 TIMING OF HCQ USE: It was striking that 100% of the 11 studies which used HCQ early in the disease on
90 an outpatient basis showed positive results. In two of the studies [9, 10] the benefit was only a trend.
91 However the effects were clinically important: in Mitja’s study resolution of symptoms was decreased
92 from 12 to 10 days; In Skipper’s study the rate of hospitalization was decreased by 60%. It is likely that
93 with higher powering statistical significance would have been reached. In the 32 other studies HCQ was
94 given on an inpatient basis with more advanced disease. The studies were divided into early, late and
95 ICU administration times. The early use, within 48 hours of admission showed 6 of 9 or 67% of the
96 studies to have positive efficacy. The two later groups, after 48 hours admission and in the ICU showed
97 2 of 5 or 40% to have positive efficacy. Thus, from 100% for early outpatient, to 67% for early hospital,
98 to 40% for later hospital use, there appears to be a relationship with time of initiation of treatment, and
99 better results the earlier HCQ is used.
100 OVERALL EFFICACY: 23 of the 43 studies (53%) showed a definite positive effect of HCQ vs COVID-19.
101 However if negatively biased studies are removed and the clinically important positive trends from
102 underpowered studies are moved to the positive efficacy group the ratio changes to 28 positive vs 9 no
103 effect: a 75% ratio of positive to non-positive HCQ studies. Interestingly none of the no-effect studies
104 showed a clear trend toward worsening.
105 RANDOMIZED CONTROLLED STUDIES (RCTs): Of the seven RCTs two [9, 10] were in the outpatient early
106 treated group. As described above both studies had clinically important trends toward positive results,
107 although were underpowered and did not reach statistical significance. The other five RCTs were in
108 hospitalized patients later in disease where efficacy seems to be less. There was 1 positive [11], 3 no
109 effect [4, 43, 44], and 1 negative effect [22] studies. The negative effect study, however, was biased, as
110 described below (“negative effect studies”), such that any negative or no-effect result would not be
111 valid. Thus two of two RCTs with early treatment showed positive results, and one of three hospitalized
112 patients had a positive result, consistent with the general finding of better results with earlier use.
113 NEGATIVE EFFECT STUDIES: Three studies had data that seemed to show worse outcomes with HCQ
114 use. All, however had significant biases. And all were in hospitalized patients when results with HCQ
115 are less good. Two [3, 16] of the three studies were well done studies that were nonetheless
116 constrained by being chartless hospitalization studies that only used billing codes at particular time
117 points to evaluate patients, but had no information as to events between these time points within their
118 hospital course which led to initiation of treatment. Both were retrospective. Patients were not
119 randomized to treatment with HCQ versus other care. Rather patients apparently received HCQ at the
120 discretion of the physician The time of administration of HCQ in the patients who received it was not
121 specified during the hospitalization. This introduces selection bias in both studies toward treatment
122 with HCQ for sicker patients who were faring worse after admission who presumably would be more
123 likely to have treatment vs no-treatment selected by their physician. Attempting to normalize
124 co-morbidities does not correct this bias because clinical progress of COVID-19 infection is not well
125 predicted by pre-existing co-morbidities. This selection basis means patients who worsened after
126 admission who are thereby more likely to have worse outcomes would be over represented in the HCQ
127 treatment group. For this reason negative results from the treatment arm of these studies are not valid
128 because outcomes are moved negatively. A positive effect however would have validity since it could
129 only occur despite the negative selection bias, not because of it.
130 The third study showing worse results with HCQ was a highly powered non-peer reviewed study whose
131 primary outcome of 28 day mortality actually showed no difference between the HCQ treated group and
132 the usual treatment group. Two of the secondary results did just barely reach significance negatively.
133 [22]. However the reporting of results was flawed as follows. 8% of the treatment group patients did
134 not receive HCQ at all; and the median number of days of treatment for all treated patients was only 6
135 out of a prescribed 9. These facts mean that less than half of patients received the full treatment
136 regimen or even two thirds of the full treatment regimen, with 1 in 12 receiving no treatment at all.
137 These untreated and undertreated patient outcomes were however grouped with the fully treated
138 patient outcomes. If HCQ has any positive effect which we believe is well established, this
139 undertreatment would invalidate their borderline negative secondary results. In addition treatment was
140 initiated more than 48 hours after admission when our aggregate data has shown a high incidence of
141 no-effect results. The study was not blinded introducing a potential undertreatment bias toward
142 patients who were known by the staff to be treated with HCQ. This study most reasonably is actually a
143 no effects study, which is common in already hospitalized patients such as these treated more than 48
144 hours after admission.
145 ADVERSE EVENTS: There have been fears among some that the increased QTc seen in some patients
146 treated with HCQ or azithromycin would predispose to Torsades de Pointes (TDP) and then death from
147 ventricular fibrillation. We found no such deaths, or death from any cause related to HCQ treatment,
148 and indeed only 1 case of TDP at all – which resolved spontaneously without treatment and without
149 sequelae. This is consistent with our prior study showing an absence of TDP mortality with HCQ use
150 [45]. All of the adverse events which seemed attributable to HCQ treatment in the 43 studies were side
151 effects known to occur with HCQ. These included nausea, vomiting, diarrhea, stomach pain, headache,
152 rash, dizziness, itching and blurred vision. In all cases there was no indication of persistence of
153 symptoms after discontinuance of the HCQ. HCQ has been used with great safety for more than 50
154 years, and the relatively minor adverse events seen in these studies is consistent with this high safety
155 profile.
156 STRENGTHS AND WEAKNESSES: A strength of this study is the large number of cohorts. A further
157 strength is the critical methodological study analysis heretofore not attempted to our knowledge for
158 these studies. One weakness is the heterogeneity of study designs which rendered comparison of study
159 results challenging. Another perceived weakness of the study could be that these include reports made
160 outside of peer-reviewed literature. Multiple papers reporting both improvement and no efficacy using
161 hydroxychloroquine that have been included in the study are either pre-prints or preliminary results of
162 larger trials. Because of the unprecedented and time sensitive nature of the SARS-COV2 pandemic the
163 scientific community has shared data and studies on a level unseen prior to this emergency. We believe
164 that these reports hold valuable information and decided to include them regardless of the way in which
165 they were published. In addition we found that both the peer-reviewed and non-peer reviewed papers
166 showed a similar breakdown between studies showing efficacy vs not so that bias was not introduced.
167 SIGNIFICANCE: We believe our findings have substantial societal global importance since there have
168 been numerous edicts either preventing HCQ use for COVID-19 or limiting it to the inpatient setting,
169 which we believe have unintentionally resulted in many unnecessary deaths. Our findings showing
170 efficacy and safety of HCQ against COVID-19 indicate that HCQ should be freely available to patients and
171 physicians who choose to use it. And it should especially be freely available to be used on an outpatient
172 basis before hospitalization where it appears to be more effective and where early fears of fatal heart
173 arrhythmias have been shown to be unfounded[45]. This is particularly important because of the other
174 drugs to show efficacy, Remdesivir, has shown no significant benefit in a recent study [46]. It is also
175 expensive and not widely available. And dexamethasone has only been shown effective in critically ill
176 hospitalized patients [47]. Convalescent plasma has shown benefit [48] but even this is not well
177 validated and plasma is not available in large numbers of doses. Thus HCQ with proven efficacy and
178 safety, a cost of 37 cents per pill and thus a total treatment cost of under 20 dollars[49], versus 3,100
179 dollars for Remdesivir[50], as well as wide supply chain availability, would appear to be the best COVID
180 19 treatment option available and needs to be widely promoted as such. Unfortunately the
181 controversies surrounding HCQ have resulted in physicians being afraid to prescribe it for reasons which
182 have nothing to do with medicine, and in patients being afraid to take it due to spurious reports of
183 danger, or fears that it is not effective. It is hoped that this study will disabuse the medical community
184 of these misapprehensions about efficacy and validate that it is both efficacious and safe, and needs to
185 be freely prescribable.
186 We also do not believe that randomized controlled studies are necessary before HCQ is authorized for
187 general use because the efficacy seen in studies already done indicates that control patients in such
188 studies might die unnecessarily; and because the time delay to do any such study would cause yet more
189 deaths by preventing HCQ use when it is most needed – which is immediately. Our study has shown
190 that good evidence of efficacy exists; and there is no safety, cost, or supply reason to not treat now.
191 Unnecessary death from delayed treatment is too high a price to pay for greater certainty of knowledge.
192 Many may have already died unnecessarily due to inaccurate HCQ information, and it is imperative that
193 we do not further add to the toll.
194 Conclusions
195 Hydroxychloroquine has been shown to have consistent clinical efficacy for COVID-19 when it is used
196 early in the outpatient setting, and in general would appear to work better the earlier it is used. Overall
197 HCQ is effective against COVID-19. There is no credible evidence that HCQ results in worsening of
198 COVID-19. HCQ has also been shown to be safe for the treatment of COVID-19 when responsibly used.
199
200 Conflict of Interest
201 The authors of this paper have no conflict of interest- financial, personal, or other in regards to COVID
202 19 or hydroxychloroquine usage. This research has received no external funding
Related:

