Source: Journal of Marine Medical Society
| Date of Submission | 23-Aug-2020 |
| Date of Decision | 18-Sep-2020 |
| Date of Acceptance | 29-Sep-2020 |
| Date of Web Publication | 06-Nov-2020 |
Correspondence Address:
Vivek Hande,
INHS Asvini, Colaba, Mumbai, Maharashtra
India
Source of Support: None, Conflict of Interest: None
DOI: 10.4103/jmms.jmms_115_20
Abstract
Background: Health-care workers (HCWs) are at high risk of acquiring COVID-19. Hydroxychloroquine (HCQ) possesses in vitro antiviral activity and inhibits viral replication of coronavirus in cell cultures. The national task force for COVID-19 in India recommended the use of HCQ prophylaxis against severe acute respiratory syndrome coronavirus 2 infection in HCWs.
Methodology: We conducted a retrospective cohort study in a mixed tertiary care facility to find the incidence and clinical profile of COVID-19 in HCWs between April 2020 and June 2020, who were advised preexposure prophylaxis with HCQ, at the start of the pandemic. Details of HCQ usage were collected using an online questionnaire form. The clinical profile, treatment, and outcome of COVID-19-positive HCWs were also studied.
Results: We included 604 HCWs, of which 491 (81.2%) had taken adequate HCQ prophylaxis while 113 (18.7%) did not take adequate HCQ, 443 (73.3%) had high-risk COVID-19 exposure, and 32 HCWs (5.1% of the total) were COVID-19 positive. There were 10 COVID-19 cases (2.1%) among HCWs taking HCQ while 22 (19.4%) cases occurred in HCQ not compliant HCWs, with a relative risk of 0.1046 (95% confidence interval: 0.0510–0.2147, P< 0.0001), indicating a reduced risk of COVID-19 among HCWs taking HCQ prophylaxis. Among the noncompliant cases, 14 (43.7%) never took HCQ, 4 (12.5%) took HCQ but had poor compliance, and 4 (12.5%) stopped HCQ prematurely. Most (91.7%) COVID-19-positive HCWs were asymptomatic or had mild symptoms, moderate symptoms were seen in 3 (9.3%), and there were no severe cases or deaths.
Conclusions: The use of HCQ as preexposure prophylaxis in HCWs was associated with reduced risk of COVID-19, suggesting its role as an effective chemoprophylactic agent.
Introduction
Health-care workers (HCWs) are particularly at a risk of acquiring COVID-19 infection due to repeated occupational exposure while working in COVID-19 facilities.[1],[2] COVID-19 in HCWs increases the risk of nosocomial transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and increases the overall disease burden.[3] A study from the United States and the United Kingdom showed that HCWs were at a 12-fold increase risk of COVID-19 positivity compared to general population.[4] The same study showed that HCWs comprised 4.8% of the total COVID-19-positive cases,[5] while in Italy, up to 20% of the HCWs were affected with COVID-19.[6]
As HCWs are exposed to a higher risk, they need additional interventions for protection against COVID-19. Adequate personal protective equipment (PPE) can mitigate the risk to some extent.[7] COVID-19 vaccine is a feasible option, but it is still in the development stages.[8] Therefore, preexposure chemoprophylaxis against COVID-19 becomes an important option to protect HCWs. Several antivirals and chemotherapeutic agents have been tried in COVID-19, but none of them have strong evidence to prove their role as a protective agent.[9]
Hydroxychloroquine (HCQ), a well-known antimalarial and immunomodulator, possesses in vitro antiviral activity and is known to inhibit viral replication of coronavirus in cell cultures.[10] This property was explored during the previous coronavirus epidemic where HCQ was used as a therapeutic option.[11] This prompted researchers to consider HCQ as a likely agent against SARS-CoV-2.[12] Moreover, HCQ prophylaxis was used successfully to avert new infections in South Korea after a large COVID-19 exposure in a long-term care facility.[13] The National Task Force for COVID-19 in India (with Indian Council of Medical Research [ICMR]) took cognizance of this evidence and empirically recommended the use of HCQ as prophylaxis against SARS-CoV-2 infection in asymptomatic HCWs treating suspected or confirmed COVID-19 cases.[14]
Based on these guidelines, all HCWs of a tertiary care hospital were advised HCQ prophylaxis from April 2020, as part of hospital policy. After few months from the onset of the pandemic in India, this study was planned to retrospectively study the incidence and profile of COVID-19 infection in these HCWs.
Study design
This is a retrospective cohort study conducted in a mixed (catering to both COVID-19 and non-COVID-19 cases) tertiary care facility in Mumbai. The HCWs advised and taking HCQ as preexposure prophylaxis were retrospectively evaluated for pattern of HCQ intake and COVID-19 infection over a period of 3 months from April to June 2020. The primary objective of the study was to find the incidence and the clinical profile of COVID-19 infections in these HCWs. The data were collected using telephone, an online questionnaire form, and by examining their medical records.
Participants
HCWs including doctors, nursing staff, paramedical staff, and supporting staff who were advised HCQ as preexposure prophylaxis as per ICMR guidelines (at dose of 800 mg on day 1 and 400 mg weekly thereafter) were included in the study. The HCWs who refused consent were excluded from the study. Requisite consent was taken from participants, wherever necessary. Requisite approval of the Institutional Ethics Committee of INHS Asvini was taken.
Methods
All HCWs included in the study were divided into two groups based on their exposure of COVID-19. The HCWs working directly in contact with confirmed cases of COVID-19 (at hospital designated facilities such as COVID-19 ward, COVID intensive care unit [ICU], and acute respiratory outpatient department), laboratory technicians working in COVID-19 laboratory and radiological technicians handling COVID-19 cases, support staff like helpers, and hygiene staff working in COVID-19 facilities, were considered a high exposure group (HEG). HCWs not included in the HEG group, but working in the mixed COVID-19 facility, were considered in the low exposure group.
The basic demographic profile of all participants was recorded including their underlying comorbidities and type of exposure. The data were collected using an online questionnaire form. The details of HCQ usage including dosage schedule, compliance, and side effects were recorded. HCWs were divided into two groups – those taking adequate dosage and compliant with HCQs and those not compliant with adequate HCQ (which included those not taking HCQ, poor compliance, or early stopping of HCQ).
HCWs with fever, cough, dyspnea, or any symptom suggestive of COVID-19; HCWs with history of unprotected exposure to COVID-19 cases; and HCWs working in COVID-19 ward/ICU after completing their duty and quarantine period, were tested for COVID-19, as per hospital policy. Swabs from nasopharynx and oropharynx were taken and tested by reverse transcription–polymerase chain reaction which used the TaqMan fluorogenic probe-based chemistry that uses the 5′-nuclease activity of Taq DNA polymerase.[15]
All COVID-19-confirmed HCWs were hospitalized and managed as per hospital protocol. The details of clinical profile and course of illness were collected from the patient records.
Statistical analysis
The data were analyzed by means of SPSS software version 20 (SPSS for Windows software; SPSS Inc., Chicago, IL, USA). The data were presented in mean, standard deviation, or median (min to max) with frequency percentage as appropriate. The HCWs taking HCQ and not taking adequate HCQ were compared in terms of number of COVID-19 cases, to calculate the relative risk. Univariate and stepwise multiple logistic regression analysis was done to find the unadjusted and adjusted odds ratio of the risk factors influencing the outcomes of HCWs. P < 0.05 was taken as statistically significant.
Results
The study included 604 HCWs who were initially advised to take HCQ starting from March 28, 2020. In July 2020, these HCWs were retrospectively evaluated for the period between April 1, 2020, and June 30, 2020. Out of these, 491 (81.2%) HCWs took adequate HCQ prophylaxis (in the recommended dose on a regular basis with good compliance) while 113 (18.7%) did not take adequate HCQ (this includes those who never started HCQ, started initially but stopped due to various reasons, or were irregular and had poor drug compliance) as shown in [Figure 1].
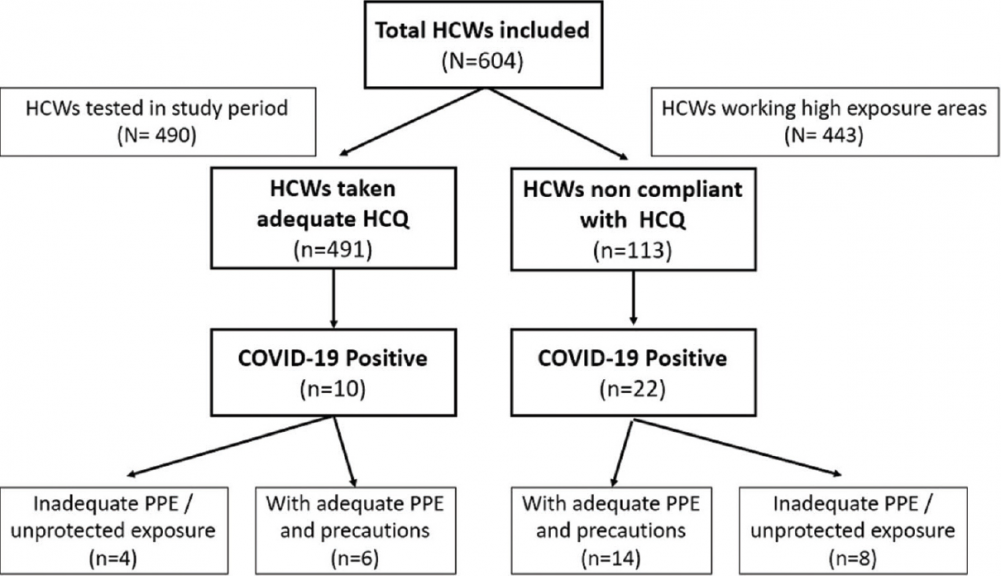
 | Figure 1: Flow diagram of the study. The flow diagram of the study shows that the total HCWs (604) included in the study were divided into HCWs who took adequate HCQ (n = 491) and HCWs who were not compliant with HCQ (n = 113). COVID-19 cases were ten among HCWs who took adequate HCQ while there were 22 cases in the group which was noncompliant with HCQ. HCWs: Health-care workers, HCQ: Hydroxychloroquine, COVID-19: SARS-CoV-2, PPE: Personal protective equipment Click here to view |
Four hundred and forty-three HCWs (73.3%) were in HEG as they were working in high-risk areas as defined earlier. Four hundred and ninety HCWs (81.1%) were tested during this period for COVID-19 which included symptomatic HCWs, those with high-risk COVID-19 exposure, and all those who worked in COVID wards. Thirty-two HCWs were found to be COVID-19 positive (5.2% of the total HCWs included in the study and 6.5% of the tested HCWs) during this period. Of these, ten cases (2.03%) occurred among HCWs who took adequate HCQ while 22 cases occurred in HCWs who were not compliant with HCQ (n = 113).
The baseline characters of the HCWs (both compliant and not compliant) with HCQ are shown in [Table 1]. Most were males, young (mean age of 33.18 ± 8.25 years), and fit with no comorbidities. There was an equal distribution of doctors, nurses, paramedical staff, and other staff. The compliance rate was 84.1% (139/162) in doctors, 85.2% (143/170) in nurses, 80.4% (136/169) in paramedics, and 70% (73/103) in other HCWs.

| Table 1: Comparison of hydroxychloroquine compliance in the different categories of health-care workers Click here to view |
The incidence of COVID-19 positivity among the HCWs is shown in [Table 2]. There were 10 COVID-19 cases (2.1%) among HCWs taking HCQ (n = 491) (exposed group), while there were 22 (19.4%) COVID-19 cases among the HCWs not compliant with HCQ (n = 113) (nonexposed group), with a relative risk of 0.1046 (95% confidence interval: 0.0510–0.2147, P < 0.0001), indicating that there was a reduced risk of COVID-19 among HCWs taking HCQ prophylaxis as compared to HCWs not compliant with HCQ.

| Table 2: Comparison of COVID-19-positive cases in groups of health-care workers compliant and noncompliant with hydroxychloroquine Click here to view |
The details of prophylactic HCQ intake were analyzed in COVID-19-positive HCWs, as shown in [Figure 2]. Among the 32 positive cases, only 10 cases (31.2%) were taking HCQ in the adequate dosage while 14 (43.7%) had not taken HCQ, 4 (12.5%) took HCQ but had poor compliance, and 4 (12.5%) HCWs initiated HCQ but stopped prematurely. It was interesting to note the pattern in different types of HCWs. In the nurses’ group, 7 (50%) were taking requisite doses, 3 (21.4%) did not take HCQ, while 4 (28.5%) stopped HCQ. Among the nurses who initially took HCQ but later stopped, three stopped because of pregnancy while one stopped because of serious dyspeptic symptoms. However, in paramedics, only two (16.6%) were taking requisite HCQ, four (33.3%) had poor compliance, and six (50%) did not take HCQ at all.

 | Figure 2: Hydroxychloroquine intake pattern and compliance in COVID-19-positive health-care workers. The figure shows the details of HCQ intake (those taking adequate HCQ, HCWs not taken HCQ, HCWs taking HCQ but having poor compliance, and HCWs initiated HCQ but stopping HCQ prematurely) among the different categories of HCWs, namely doctors, nurses, paramedics, and other support staff. HCWs: Health-care workers, HCQ: Hydroxychloroquine, COVID-19: SARS-CoV-2 Click here to view |
The clinical characteristics of COVID-19-positive HCWs compliant with HCQ (n = 10) and not compliant with HCQ (n = 22) were compared, as shown in [Table 3]. Among various types of HCWs, nurses (n = 10) were predominant among COVID-19-positive HCWs taking HCQ (70%), while paramedical staff (n = 10) were predominant in the group not taking HCQ (45.4%). Most of these were working in COVID-19 wards or other high-risk exposure areas. The incidence of HCWs who had unprotected exposure to COVID-19 cases was equal in both the groups, with four (40%) of HCWs in the HCQ compliant group and eight (36.3%) of HCWs in the HCQ noncompliant group.

 | Table 3: Clinical characteristics of COVID-19 in health-care workers compliant with hydroxychloroquine and health-care workers noncompliant with hydroxychloroquine Click here to view |
Most COVID-19 cases were asymptomatic: 5 (50%) and 13 (59%) in HCWs compliant and in HCWs not compliant with HCQ, respectively. Mild symptoms were seen in four (40%) and eight (36.3%) in both the groups, respectively, and only one (10%) and two (9%) had moderate symptoms in both the groups, respectively. There were no critically ill or severe COVID-19 cases among the HCWs, and no mortality occurred. The common clinical symptoms and signs in both the groups were similar, as shown in [Table 3]. The treatment given was as per the protocols at that time of the pandemic; HCQ was administered to all COVID-19 cases, azithromycin and lopinavir/ritonavir to cases with moderate symptoms. Oxygen was used in moderate cases: one (10%) in the HCW compliant group and two (9%) in the HCW noncompliant group.
Univariate logistic regression analysis was done to evaluate the factors associated with taking or not taking HCQ in COVID-19-positive HCWs. The factors analyzed were sex, being a nurse, being a paramedical staff, working in high exposure areas, inadequate PPE, and presence of COVID-19 symptoms, but none were found to be statistically significant.
Discussion
HCQ has been the most studied drug for chemoprophylaxis of COVID-19 with both positive and negative outcomes emerging from different studies.[16],[17] The present study highlights the use of HCQ preexposure prophylaxis as an institutional policy. The use of HCQ as preexposure prophylaxis in HCWs was associated with reduced risk of COVID-19 (2.1%) compared to HCWs not taking adequate HCQ (19.4%), with both the groups being demographically similar in all respects and having similar levels of exposure. The total incidence of COVID-19 in HCWs (32/604) (2.1%) in this study was lower than the incidence of COVID-19 in HCWs in previous studies in India (5.01%) (1073 of 21402 HCWs screened being COVID-19 positive)[7] and other countries like The Netherlands (5%) (96 of 1796 HCWs)[3] at that time of the pandemic.
Similar results were also seen in a cohort study in HCWs in Kolkata (n = 106) which found that the use of HCQ was associated with reduced incidence of COVID-19 compared to HCWs not on HCQ, which was statistically significant (Chi-square = 14.59, P < 0.001).[18] In a case–control study by ICMR, 23,898 HCWs were selected from countrywide COVID testing data portal, and the details of their HCQ intake were analyzed.[7] It was seen that there was a higher risk of COVID-19 infection associated with lack of HCQ prophylaxis on univariate analysis (P = 0.087), and taking four or more doses of HCQ prophylaxis was associated with a significant decline in risk of COVID-19 infection.[7] Postexposure prophylaxis of HCQ has also been studied in 821 patients, but there was no difference in the development of COVID-19 infection in those who received postexposure HCQ prophylaxis within 4 days of exposure (49 of 414 [11.8%]), compared to those who received placebo (58 of 407 [14.3%]).[19]
The potential antiviral and anti-inflammatory properties of HCQ, together with the low cost of therapy, good oral bioavailability,[20] high tissue concentrations in the lungs relative to the plasma levels, and acceptable safety profile, favor its use for preexposure chemoprophylaxis.[10]
HCQ exerts its in vitro anti-COVID-19 effects by various ways.[21] HCQ increases lysosomal pH and inhibits endosomal acidification, thus preventing viral entry into host cells.[22] HCQ inhibits terminal glycosylation of angiotensin-converting enzyme 2 (ACE-2) receptor causing reduced binding efficiency between ACE-2 on host cells and the SARS-CoV-2 spike protein, thus inhibiting viral entry and replication.[23],[24] HCQ also stops the release of viral genome by blocking the transport of SARS-CoV-2 from early endosomes to early lysosomes.[10],[25] In spite of in vitro success in inhibiting SARS-CoV-2, clinical trials have failed to show any benefit of HCQ as a therapy.[26]
Few studies have shown the lack of benefit of HCQ as a therapeutic agent, and there have been safety concerns associated with HCQ use.[16] This apparent disparity with the findings of the current investigation could be explained by the fact that in treatment settings, severe COVID-19 patients are likely to have a very high viral load and cytokine levels, which may not be improved by HCQ therapy.[27] Moreover, the toxicities of HCQ are less likely to occur when taken as prophylaxis as it is used at a low weekly dose.
In this study, 491/600 (81.4%) of HCWs took HCQ in adequate dosages. A study from New Delhi found that 76% of the HCWs agreed to take HCQ prophylaxis and 85% of these showed good adherence.[28] The HCWs with COVID-19 were predominantly nurses (43.7%) and paramedical staff (37.5%). Nurses were most commonly affected HCWs, as in a study from Wuhan,[29] with nurses (56.4%) followed by health-care assistants (20%), and in another Chinese study which showed that 52.06% of the HCWs were nurses.[30] Most of the HCWs in this study were asymptomatic (56.25%) or had a mild illness (33.33%) with none having severe disease or mortality. Other studies also showed that the HCWs had a less severe disease than other COVID-19 patients (9.9% vs. 29.4%, P < 0.001)[31] and nonsevere cases comprised 84.5% of the total affected HCWs.[29] The case fatality rate of HCWs has been <1% in most studies.[30],[31]
Conclusions
The strength of the study is that it is an institutional-based study where HCQ was recommended and provided to all HCWs, and all HCWs could be followed up for 3 months. In the absence of strong clinical trials on the safety and efficacy of HCQ preexposure prophylaxis, this study offers evidence, which is of public health importance.
The main limitations of the study are its small sample size and nonrandomized retrospective observational design, due to which a definite cause and effect relation cannot be established. Moreover, it is possible that the study results may have been influenced by several confounding factors which could not be measured. A large-scale multicentric prophylactic randomized trial is required to establish the role of HCQ prophylaxis.
The use of HCQ as preexposure prophylaxis in HCWs was associated with reduced risk of COVID-19, suggesting its role as an effective chemoprophylactic agent. Therefore, in the absence of robust data in the present pandemic scenario, we recommend continued use of HCQ prophylaxis for COVID-19 in HCWs, based on the ICMR guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
| 1. | Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, et al. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res 2020;151:147-59. [PUBMED] [Full text] |
| 2. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199-207. |
| 3. | Sikkema RS, Pas SD, Nieuwenhuijse DF, O’Toole Á, Verweij J, van der Linden A, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: A cross-sectional study. Lancet Infect Dis 2020;S1473-3099(20)30527-2. |
| 4. | World Health Organization. Coronavirus Disease (COVID-19) Situation Report-167. World Health Organization; 2020. |
| 5. | Nguyen LH, Drew DA, Joshi AD, Guo C-G, Ma W, Mehta RS, et al. Risk of symptomatic Covid-19 among frontline healthcare workers.a prospective cohort study. Lancet Public Health 2020;Jul 30:S2468-2667 (20) 30164-X. |
| 6. | The Lancet. COVID-19: Protecting health-care workers. Lancet 2020;395:922. |
| 7. | Chatterjee P, Anand T, Singh KJ, Rasaily R, Singh R, Das S, et al. Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19. Indian J Med Res 2020;151:459-67. [PUBMED] [Full text] |
| 8. | Nagesh S, Chakraborty S. Saving the frontline health workforce amidst the COVID-19 crisis: Challenges and recommendations. J Glob Health 2020;10:010345. |
| 9. | Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA 2020;323:1824-36. |
| 10. | Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery 2020;6:16. |
| 11. | Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;22;2:69. |
| 12. | McCaw JM, McVernon J. Prophylaxis or treatment? Optimal use of an antiviral stockpile during an influenza pandemic. Math Biosci 2007;209:336-60. |
| 13. | Lee SH, Son H, Peck KR. Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals? Int J Antimicrob Agents 2020; June 55(6):105988. |
| 14. | Advisory on the use of Hydroxychloroquin as Prophylaxis for SARS CoV2 Infection. By National Task Force for COVID-19. Ministry of Health & Family Welfare, Government of India; 22 March, 2020. Available from: https://www.mohfw.gov.in. |
| 15. | Advisory Newer Additional Strategies for COVID-19 Testing Existing Strategies for COVID-19 Testing. Indian Council of Medical Research. Available from: https://www.icmr.gov.in/pdf/covid/strategy/Advisory_for_rapid_antigen_test_14062020.pdf. [Last accessed on 2020 Aug 01]. |
| 16. | Das S, Bhowmick S, Tiwari S, Sen S. An updated systematic review of the therapeutic role of hydroxychloroquine in coronavirus disease-19 (COVID-19). Clin Drug Investig 2020;40:591-601. |
| 17. | Bienvenu AL, Marty AM, Jones MK, Picot S. Systematic review of registered trials of Hydroxychloroquine prophylaxis for COVID-19 health-care workers at the first third of 2020. One Health 2020;10:100141. |
| 18. | Bhattacharya R, Chowdhury S, Mukherjee R, Nandi A, Kulshrestha M, Ghosh R, et al. Pre-exposure Hydroxychloroquine use is associated with reduced COVID19 risk in healthcare workers. medRxiv 2020;06.09.20116806. |
| 19. | Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020;383:517-25. |
| 20. | Zhou D, Dai SM, Tong Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother 2020;75:1667-70. |
| 21. | Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect Dis 2003;3:722-7. |
| 22. | Al-Bari MA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect 2017;5:e00293. |
| 23. | Li W, Moore MJ, Vasllieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-4. |
| 24. | Freund NT, Roitburd-Berman A, Sui J, Marasco WA, Gershoni JM. Reconstitution of the receptor-binding motif of the SARS coronavirus. Protein Eng Des Sel 2015;28:567-75. |
| 25. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271-80.e8. |
| 26. | Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv 2020;04.16.20065920. |
| 27. | Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents 2020;55:105982. |
| 28. | Bhattacharyya D, Raizada N, Nagappa B, Tomar A, Maurya P, Chaudhary A, et al. Chemoprophylaxis of COVID-19 with hydroxychloroquine: A study of health care workers attitude, adherence to regimeand side effects. medRxiv 2020;06.11.20126359. |
| 29. | Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open 2020;3:e209666. |
| 30. | Zheng L, Wang X, Zhou C, Liu Q, Li S, Sun Q, et al. Analysis of the infection status of the health care workers in Wuhan during the COVID-19 outbreak: A cross-sectional study. Clin Infect Dis 2020;ciaa588. |
| 31. | Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in health care workers – A systematic review and meta-analysis. Am J Emerg Med 2020;38:1727-31. |
Related: HCQ is effective for COVID-19 when used early: analysis of 132 studies