Source: MedRxIV
David M. Wiseman, Pierre Kory, Samir A Saidi, Dan Mazzucco
doi: https://doi.org/10.1101/2020.11.29.20235218
Abstract
BACKGROUND A recent trial (NCT04308668) found that post-exposure prophylaxis with hydroxychloroquine (HCQ) was associated with a reduced incidence of Covid-19 by 17% overall; 36% in younger subjects, 31% in household contacts and 49% given within one day. To understand these trends, we prospectively re-analyzed the released dataset.
METHODS Our protocol conformed to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT). We compared the incidence of Covid-19 after HCQ or placebo, stratifying primarily by time to drug receipt, age and gender.
RESULTS Requesting additional data, we found that 52% of subjects received medication 1-2 days after the intended overnight delivery; 19% of them outside the intended four-day window from exposure. After re-analysis, there was a reduced incidence of Covid-19 associated with HCQ compared with placebo (9.6% vs. 16.5%) when received up to 3 days (Early) after exposure (RR 0.58, 95%CI 0.35 – 0.97; p=0.044; NNT 14.5) but not later (Late) (RR 1.22, 95%CI 0.72 – 2.04).
We found a significant HCQ-associated reduction in subjects 18 to 45 years old associated with Early (RR 0.53, 95%CI 0.29-0.97; p=0.0448, NNT 11.5) but not Late (RR 1.02, 95%CI 0.55-1.89) prophylaxis, attenuated in older subjects (RR 0.75, 95%CI 0-27-2.05) and by co-morbidities. There were reductions associated with Early prophylaxis in household contacts (RR 0.35, 95%CI 0.13-0.89; p=0.025, NNT 5.7) and Health Care Workers (RR 0.74, 95%CI 0.4-1.38). We did not detect effects of gender, folate, zinc, or ascorbic acid.
CONCLUSIONS Using novel data with a prospective post hoc re-analysis, hydroxychloroquine, in an age-dependent manner, was associated with reduced illness compatible with Covid-19 or confirmed infection when supplied for post-exposure prophylaxis between 1 and 3 days after high-risk or moderate-risk exposure. This finding warrants prospective confirmation.
Registered with the Open Science Framework (last revised September 27, 2020, osf.io/fqtnw).
Plain Language Summary A recent clinical trial examined the ability of hydroxychloroquine (HCQ) to prevent Covid-19 just after an exposure to a person confirmed to have Covid-19. There was an HCQ-associated reduction of Covid-19 by an overall 17%; 36% in younger subjects, and 49% in subjects given HCQ within one day of being exposed. Likely because the study had too few patients and was designed to find a larger overall difference, this effect was not statistically significant, even though it may have been medically and economically meaningful.
When we studied the trial data, we found an unintended, unknown and variable delay in the delivery of study drug which may have masked any effect of HCQ. The investigators provided further information at our request that confirmed our theory; about half of the participants received their drugs one or two days later than intended. About a fifth of them received their drugs beyond the latest time (four days) the investigators thought the drug might work.
When we factored in this new information, we found that if HCQ was given early (up to three days after exposure), it was associated with a 42% reduction of Covid-19, which was statistically significant. Giving HCQ later had no effect. There was a greater effect in younger (less than 45 years) rather than older subjects (47% vs. 25%). Gender did not seem to affect the results, but there was a greater HCQ-associated reduction (65%) when it was given early to people exposed to Covid-19 in a household environment rather than to health care workers (26%). The effects associated with HCQ were better in people who did not have co-existing conditions.
These re-calculations are important because the study, as originally analyzed, was one of only four randomized studies cited by FDA to support a key public health decision made in June 2020 regarding HCQ. It was the only randomized study cited that dealt specifically with the question of whether the drug could prevent Covid-19. Although other research has shown that the drug is not effective to treat well-established cases of Covid-19, our research suggests that that it might be effective when used for prevention. This paves the way for our result to be confirmed under clinical trial conditions and for a re-examination of public health policy regarding this drug.
Short Summary A prospective re-analysis of a public dataset integrated with novel data found that hydroxychloroquine was associated with reduced illness compatible with Covid-19 when received between 1 and 3 days after a high-risk or moderate-risk exposure (RR 0.58, 95% CI 0.35-0.97, p=0.044, NNT14.5).
Introduction
There have been (as of November 29, 2020) over 61.8 million cases of Covid-19 and over 1.4 million deaths worldwide,1 about one fifth of them within the USA.2 Early interest developed in deploying hydroxychloroquine (HCQ) and in March 2020 the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA).3 Lacking randomized clinical trial (RCT) data, many observational reports emerged which were unfavorable (with exceptions4) to the use of HCQ.5 With toxicological concerns, on April 24, FDA cautioned6 against using HCQ outside hospital or trial settings.
HCQ became highly controversial, it being suggested that “to some extent the media and social forces — rather than medical evidence — are driving clinical decisions and the global Covid-19 research agenda.”7 Against this background, on June 15, FDA revoked3 HCQ’s EUA, citing only two just-published substantive RCTs. The findings of the RECOVERY Trial8 announced June 5 was cited as offering “persuasive evidence of a lack of benefit of HCQ in the treatment of hospitalized patients.”
The second and only study,9 addressing prevention examined post-exposure prophylaxis (the “PEP” study) with HCQ in 821 asymptomatic adults with a high or moderate risk household or occupational exposure to Covid-19. Subjects received HCQ (1.4g first day, then 600 mg daily for 4 more days) or folate (USA) or lactose (Canada) placebo. The study concluded that “…HCQ did not prevent illness […] when initiated within 4 days after […] exposure” (HCQ 11.8% vs. placebo 14.3%; RR 0.83, 95%CI 0.58-1.18, p=0.35).
We10 and others have criticized the study’s interpretation. Since this was a pragmatic trial, with typically greater heterogeneity and smaller effect sizes than in an explanatory trial,11 powering the study to detect a 50% reduction in Covid-19 may have been over-ambitious, especially given its early termination.12 An arguably13 clinically meaningful reduction of 17% was similar to that associated with dexamethasone.14 The authors suggested15 that the study was primarily powered to collect data quickly under pandemic conditions rather than to meet specific clinical goals.
Non-statistically significant signals of HCQ-associated efficacy included an overall effect apparently driven by a 31% reduction among household cohabitees. There were age-dependent reductions (<= 35 years, 36%) found in other analyses16 to be statistically significant. The use of folate as placebo and the ex-protocol use of zinc and Vitamin C may have been confounding (Supplement). With a reduction of 49% associated with early (“Day 1”) HCQ prophylaxis, we10 and others17 found a negative association between treatment lag and reduction of Covid-19.
We conjectured that post hoc exploratory re-analysis of the PEP study dataset would inform a time- and age-nuanced approach to Covid-19 using HCQ, testable in prospective studies. Our objectives were to define: (a) any time-dependent, or (b) any age-dependent effects associated with HCQ and, (c) any influence of gender, exposure type, use of zinc, ascorbic acid or folate on the study outcomes.
Methods
Dataset and Protocol Revisions
One protocol (NCT04308668) described separately reported post-exposure prophylaxis (PEP)9 or early post-exposure treatment (PET)18 cohorts. The de-identified PEP dataset was released (covidpep.umn.edu/data) with three revisions: September 9 (“9/9”), October 6 (“10/6”) and October 30 (“10/30”) 2020.’’
Using the Open Science Framework (OSF) protocol template (osf.io/jea94/), we conformed to the SPIRIT checklist (Standard Protocol Items: Recommendations for Trials19) and integrated the WHO Trial Registration Data Set.20 Our protocol was registered on August 13, 2020 with revisions (Supplement), most recently September 27, 2020 (osf.io/vz8a7/10) prior to receiving data regarding the time to drug receipt in the 10/6 revision.
There were four main areas requiring clarification (Supplement) related to: (i) definition of exposure risk; (ii) identification of subjects adhering to study medication; (iii) estimation of the interval from exposure to receipt of medication (resolved with new data in the 10/6 revision); and (iv) nomenclature describing timing of study events counting the date of highest reported exposure to Covid-19 as “Day 1.” Adopting this clarification, we note some inconsistencies with the original paper indicating the occurrence of study events to be one day later.
Analysis Plan
We re-stratified data by time to drug receipt before further stratification by age, gender, type of exposure, risk type, or use of zinc or ascorbic acid. An Intent-to-Treat (ITT) analysis was employed as in the original study. We analyzed data according to adherence to taking study medication, whether subjects provided outcome data, the use of the folate placebo, and presence of co-morbidities (Supplement).
We used the same primary outcome variable as the original study: “incidence of either laboratory-confirmed Covid-19 or illness compatible with Covid-19 within 14 days.” The incidence of Covid-19 in subjects treated with HCQ and placebo were compared using Fisher’s Exact test. We examined the severity of symptoms at 14 days according to a visual analogue scale described as a secondary outcome in the original study (Kruskal-Wallis test).
We mirrored the use in the PEP study of two-tailed tests without adjustment for multiple comparisons. This is further justified by the exploratory nature of our analyses. p-values ≤ 0.05 were considered statistically significant. Larger values are presented to identify trends. Microsoft Excel was used for data processing. Vassar Stats (vassarstats.net/) was used for verification. The original authors provided two calculations from which we were able to verify our primary time stratification (Supplement).
Ethics Committee Approval
No ethics committee approval was required as we used a de-identified, publicly released dataset.
Results
Considering shipping schedules, we estimated10 that within each of the reported strata for “Time from exposure to enrollment” (1 to 4 days), there were overlapping variations in time from exposure to drug receipt. For example, some “Day 1” (range 1.4-4.4 days) and “Day 4” (range 4.4-7.4 days) subjects may have received drug after the same interval.
New data (9/9 revision) provided at our request broadly confirmed these estimates revealing a reduction in Covid-19 associated with HCQ given within 2 (elapsed time) days of exposure (RR 0.35, 95%CI 0.13 – 0.93; p=0.0438) but not later (RR 0.98, 95%CI 0.67 – 1.45).10 Recognizing limitations (Supplement) to these estimates, further detail was requested and provided (10/6 revision). The PEP study protocol had intended to enroll only those receiving drug within 4 days from exposure, assuming overnight shipping. We found that 332 and 95 subjects (52% of all subjects) received medication one or two days later than this respectively, with 152/821 (19%) subjects receiving drug outside of the intended 4-day window (Table S 3).
We stratified subjects according to the interval between exposure and drug receipt. We found a reduction in Covid-19 associated with HCQ when received “Early” between 1 and 3 (elapsed time) days after exposure from 16.5% to 9.6% (RR 0.58, 95%CI 0.35 – 0.97; p=0.044; NNT 14.5) but not after three days (“Late”) (RR 1.22, 95%CI 0.72 – 2.04) (Table 1). We did not detect differences in the severity of symptoms for both Early and Late prophylaxis cohorts (Supplement). A comparison of the demographic and clinical characteristics of the two groups shows a largely conserved balance between the Early and Late cohorts (Table 2, Table S 1).
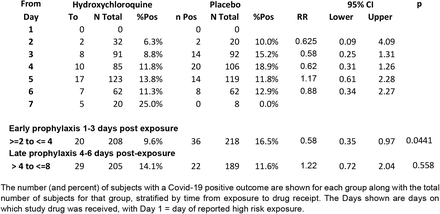
Table 1: Stratification of effect associated with hydroxychloroquine based on time from exposure to drug receipt (ITT population)

Table 2: Demographic and clinical characteristics, stratified into Early and Late Cohorts The data for the original cohort recreates data from the original paper, for comparison and quality control purposes. Several variables have been added. The data are stratified into Early (1-3 days) and Late (4-6 days) post exposure prophylaxis cohorts. (I/S/%) – Shown in parentheses are interquartile ranges (1st and 3rd quartile), or standard deviations where indicated. All other values within parentheses indicate the percent contribution to the cohort total. See Table S 1 for full list of demographic and clinical characteristics.

Table 3: Stratification of effect associated with hydroxychloroquine by age based on time from exposure to drug receipt (ITT population)
Adopting the same age strata as the PEP study, we found in the Early cohort non statistically significant Risk Ratios of 0.53 (18-35 years), 0.52 (36-50 years), and 2.80 (> 50 years). With no a priori reason for selecting these strata, the data are less subjectively supportive of two age strata. We discerned a boundary between 42 and 48 years and on a post hoc exploratory basis we set it at the point where the upper 95% confidence interval for one of the Risk Ratios in the two strata was <1. We found reductions of Covid-19 associated with drug given up to 3 (elapsed time) days after exposure in both younger (18-45 years) (RR 0.53, 95%CI 0.29-0.97; p=0.0448, NNT 11.5) and older (>45 years) (RR 0.75, 95%CI 0-27-2.05) subjects. Within the Early and Late cohorts, further stratification revealed no obvious gender-based differences in this effect (Table S 4).
Considering only subjects reporting no co-morbidities (particularly excluding asthma and co-morbidities classified as “other”), suggested stronger effects associated with HCQ within time- or age-related strata (Supplement).
With a higher baseline rate in household contacts (26.8%) than in HCW (13.8%), the reduction of Covid-19 in household contacts associated with Early HCQ was statistically significant, compared with placebo (RR 0.35, 95%CI 0.13-0.89; p=0.025, NNT 5.7)(Table 4).

Table 4: Stratification of effect associated with hydroxychloroquine by exposure type based on time from exposure to drug receipt (ITT population)
Directionally similar results were obtained after excluding subjects who did not contribute to outcome data and subjects who did not take study medication. Stratifying into Early and Late prophylaxis cohorts revealed no discernible effect associated with folate (Supplement). With poorly detailed observational data, there did not appear to be an effect associated with zinc or Vitamin C (Supplement). The use of zinc and ascorbic acid appears balanced between the groups both for the whole cohort and the Early and Late time strata (Table 1).
Discussion
In tackling our primary objective of defining any temporal effect of HCQ, our assumption was that HCQ prophylaxis had been “initiated within 4 days after […] exposure.”9 We were joined in this understanding by others,13,16,17 including the authors of NIH21 guidelines and the editorial7 accompanying the paper. This assumption requires revision for two main reasons.
Firstly, many participants received medication after the intended overnight delivery or after four days from exposure. A similar issue likely pertains to the companion PET study.18 Secondly, inconsistent terminology lead to an overestimate by one day of the time from exposure to enrollment or to drug receipt.
Correcting these assumptions results in a statistically significant reduction of 42% of Covid-19 associated with HCQ when received between 1 and 3 (elapsed time) days after exposure, with no effect later than this. The early use of HCQ is supported by estimates for the incubation period of 3-8 days22
We found an age-dependent, statistically significant reduction of Covid-19 associated with drug given Early. This finding is consistent with a 45% reduction of Covid-19 associated with HCQ pre-exposure prophylaxis (PrEP)23 in younger patients (HR 0.55, CI 0.32-0.96, p=0.038, combined treatment groups) in a companion study and an observational study involving mainly younger HCW.24 Apparent age-dependent and time stratified effects associated with HCQ may be confounded by increases in incubation period with age.25 Re-analyzing the same PEP dataset without time stratification and confirmed by us (Supplement), Luco16 described reductions in Covid-19 associated with HCQ in subjects younger than 50 years (RR 0.71, p=0.089) reaching statistical significance (RR 0.63, p=0.029) in the sub-cohort experiencing high-risk exposures.
Small population sizes within the co-morbidity subgroups prompt cautious interpretation. However, the presence of co-morbidities attenuated age- and time-dependent effects associated with HCQ. Although age-related responses associated with HCQ may be dependent on the presence of co-morbidity,16 excluding patients with co-morbidities did not yield equivalent effects in the younger and older age strata. Asthma and co-morbidities classified as “other” contributed most to attenuating the response associated with HCQ. This finding is supported by the application of Multiple Correspondence Analysis and the Mantel test to the same dataset by Luco16 who described confounding clinical differences, between study groups particularly regarding asthma and “other” co-morbidities.
Mirroring the original data, we found a significant effect associated with HCQ in household contacts. This result may reflect differences in access to advanced PPE, hygiene training, and the ability to quarantine after exposure. A further difference may originate from the limitation discussed relating to likely multiple high-risk exposures in household contacts. Thus, household contacts in this study may share much with the first responders in the companion PrEP study,23 where a 64% reduction in Covid-19 associated with HCQ was observed (combined dose groups).
We did not detect an effect of using folate. Due a paucity of data, we could not determine whether there was an effect of zinc or Vitamin C other than noting no differences associated with HCQ in subjects taking neither agent and the entire ITT cohort. Further, based on the apparently balanced use of zinc and Vitamin C in the stratified cohorts, confounding due to these agents seems unlikely.
Our findings are made in the general climate of concern26 for the reliability of publications related to Covid-19 and the continuing controversy and confusion surrounding HCQ.27,28 This is partly fueled by a widening understanding of Covid-19 pathogenesis and the multiple mechanisms that have been proposed for HCQ.29 It is important therefore in discussing HCQ’s putative actions to do so in relation to a specific stage in the disease.
In hospitalized patients RCT findings30 evincing HCQ’s ineffectiveness are supported by observational studies, notably two related studies4,31 reporting a significant reduction in mortality associated only with HCQ’s use with zinc. Further confounding within a number of observational studies is related to the possibly synergistic use of steroids.32 At earlier stages, any effect associated with HCQ appears independent of zinc, evinced by the lack of synergy we observed between HCQ and zinc. Further, using zinc may be futile in otherwise healthy, especially younger subjects with no zinc deficiency or dysregulation.
For prophylaxis, understanding differences in timing of intervention, patterns of exposure, the ability to quarantine, testing methods, co-morbidities, and the possibility of multiple rather than single “index” exposures, appear important in reconciling apparently conflicting studies where distinctions between pre- and post-exposure prophylaxis may become blurred.
Our findings are consistent with those of a prospective, non-randomized, study33 in which asymptomatic subjects (non-HCW, mean age 37.2 years) mostly exposed to laboratory confirmed COVID-19 cases could opt to receive HCQ prophylaxis (800 mg first day, then 400 mg weekly for 3 weeks) or standard care alone (control). There was a reduction of the 4-week incidence of Covid-19 associated with HCQ compared with controls (19.4% vs. 10.6%, p=0.033, NNT =12). A single “index” exposure could not be identified. Nonetheless, subjects were enrolled after the primary case positive report and prophylaxis began at the earliest within 48 hours of knowing about the high-risk contact (D. Dhibar personal communication).
A household randomized trial34 reported in abstract form examined the effect of HCQ given to subjects last exposed to an infected person within 96 hours of enrollment. By day 14, no difference in infection rate was observed between the HCQ and ascorbic acid control groups (aHR 0.99, 95% CI 0.64-1.52). Medication was mailed with an unstated shipping time, and data were neither stratified by age nor time from exposure to drug receipt.
A Spanish cluster-randomized trial35 found a small reduction of Covid-19 associated with HCQ (RR 0.86, 95%CI 0.52-1.42). There was a higher mean age (∼ 48 years) than in the PEP study (∼ 42 years) where we have suggested a stratum boundary at approximately 45 years. There was a similar effect associated with intervention <= 3 days (RR 0.89) or 4-6 days (RR 0.93), but not >= 7days (RR 4.09) after exposure. Notably, there were differences according to a subject’s status by Covid-19 PCR testing at baseline. In PCR-positive subjects there was no effect associated with HCQ (RR 1.02; 95%CI 0.64–1.63), whereas for PCR-negative patients there was a signal for an HCQ-associated effect (RR 0.68, 95%CI 0.34–1.34). A change in PCR status is likely to be a function of the moment beyond which a drug is unlikely to be effective, possibly more accurately than an estimate of time from exposure. Accordingly, this study supports our findings suggesting a beneficial effect associated with HCQ when used early.
Further, the PCR-negative cohort from the Spanish study shares much with not only the PEP study population, but also that of its companion PrEP study.23 Although the PrEP study was hampered by poor recruitment, once or twice weekly use of HCQ in HCW was associated with reduced development of Covid-19 by 27%, compared with folate placebo (HR 0.73, CI 0.48-1.09, p=0.12, combined groups). Comparison with another PrEP RCT36 in HCW, with a very small study population (n=132) due to early termination and a low incidence of Covid-19, is not meaningful.
Limitations
Our primary time stratification based on newly-acquired data essentially represents the a priori analysis intended by the original PEP study. Nonetheless, this remains a post hoc study, and our examination of various subgroups is subject to well-known statistical challenges.37 These are partially offset by our stringent use of two-sided tests, when directionality in the original data may have justified using one-sided tests. Results should be interpreted cautiously, and the hypotheses generated should be tested in prospective studies sufficiently powered to accommodate multiple comparisons in sub-groups.
Our study retains the limitations acknowledged by its original authors related to the availability and access to testing, the use of a clinical case definition of Covid-19, the reliance on self-reported data and the generally young population studied. There are other limitations. The study poorly represents African-American and Hispanic or Latino populations. The rapidly executed study overcame several logistical challenges to allow the collection of real-world data, having both advantages and disadvantages of a pragmatic design.11,38 Self-selection bias inherent in this type of study may have been compounded by FDA cautions regarding HCQ.6 Unlike similar studies,34,35 the original PEP study was not cluster-randomized.38
Several limitations relate to the estimation of the interval between exposure and treatment with 24-hour windows of uncertainty on either side. The earlier of these is due to subjects providing only the date of their highest risk exposure. The later window is due to the way the drug receipt times were de-identified, and the unknown interval before the ingestion of the first dose. The original authors (personal communication) attempted to ensure that this was minimized by delivering medication to an address where the participant knew they would be at its expected arrival time. Rather than a single “index” exposure, there were likely other exposures before the “index” contact was diagnosed.
Time-related or other biases may be associated with the exclusion of 100 subjects who were randomized to the PEP study, became symptomatic before medication was received and aggregated into the companion treatment study.18
The poorly understood relationship between age and susceptibility to Covid-19 provides no a priori justification for defining particular strata especially in a weakly powered and statistically fragile system. Indeed, the same three strata were used in one companion study18 to the PEP study but not another.23 Several methods39 have been proposed to define strata which we attempted to accomplish empirically on a post hoc exploratory basis. Lastly, analysis of the effect of risk level is confounded by its changing definitions and the inability to discriminate between nuances within the high-risk category.
Conclusions
Analysis40 of the PEP,9 companion23,18 and other35 studies raise no significant safety concerns for using HCQ in the populations studied. Integrating a public dataset with new unpublished data, we found that, especially in younger subjects, hydroxychloroquine was associated with significantly reduced illness compatible with Covid-19 when initiated between 1 and 3 days after a high-risk or moderate-risk exposure. This finding warrants prospective confirmation.
Data Availability
Microsoft Excel files will be made available on request to other investigators up to one year after publication. The original dataset is available from the authors of the original study via their study web site: covidpep.umn.edu/data
Funding and Conflicts of Interest
There is no external support for this study. The sponsor is entirely responsible for the design and conduct of this study. The sponsor and principal investigator have no financial or other conflicts of interest in the subject matter of this protocol. DMW is the president of Synechion, Inc., that provides services for the medical industry outside of the area of this work. DMW is also the president of KevMed, LLC., that markets medical products outside the scope of this work. DM is the president of ZSX Medical, LLC. that develops surgical devices and a Principal at Third Eye Associates, a technical consulting company. PK and SAS report no conflicts.
Data Sharing
Microsoft Excel files will be made available on request up to one year after publication.
Acknowledgements
We thank Dr. David Boulware and his colleagues for clarifying our questions, providing insight into their study, collecting additional data at our request related to shipping times and providing confirmatory calculations for our analysis. We also thank Drs. Marcio Watanabe, Juan Luco and Philip Lavin for their helpful comments. This acknowledgment does not imply endorsement of our work.
References
- 1.↵World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. (Accessed Nov 29, at https://covid19.who.int/.)Google Scholar
- 2.↵Centers for Disease Control and Prevention. CDC COVID Data Tracker. 2020. (Accessed Nov 29, at covid.cdc.gov/covid-data-tracker/.)Google Scholar
- 3.↵Hinton D. Food and Drug Administration. Letter to Dr. GL Disbrow (BARDA), revoking EUA for hydroxychlroquine, June 15. 2020. (Accessed Nov 11, 2020, at https://www.fda.gov/media/138945/download.)Google Scholar
- 4.↵Carlucci P, Ahuja T, Petrilli CM, et al. Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. medRxiv 2020:2020.05.02.20080036. Epub May 8 http://doi.org/10.1101/2020.05.02.20080036Google Scholar
- 5.↵Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020. Epub May 8 http://doi.org/10.1056/NEJMoa2012410Google Scholar
- 6.↵FDA. FDA Drug Safety Communication: FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems 2020 April 24. (Accessed July 16, 2020, at https://www.fda.gov/media/137250/download.)Google Scholar
- 7.↵Cohen MS. Hydroxychloroquine for the Prevention of Covid-19 – Searching for Evidence. N Engl J Med 2020 June 4. Epub June 4 http://doi.org/10.1056/NEJMe2020388Google Scholar
- 8.↵Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. June 5 2020. (Accessed June 16, 2020, at https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf.)Google Scholar
- 9.↵Boulware DR, Pullen MF, Bangdiwala AS, et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med 2020. Epub 2020 June 4 http://doi.org/10.1056/NEJMoa2016638Google Scholar
- 10.↵Wiseman DM, Kory P, Mazzucco D, Ramesh MS, Zervos M. Treatment and prevention of early disease before and after exposure to COVID-19 using hydroxychloroquine: A protocol for exploratory re-analysis of age and time-nuanced effects: Update based on initial dataset review. medRxiv 2020:2020.08.19.20178376. Epub Oct 9 http://doi.org/10.1101/2020.08.19.20178376Google Scholar
- 11.↵Shortreed SM, Rutter CM, Cook AJ, Simon GE. Improving pragmatic clinical trial design using real-world data. Clinical trials (London, England) 2019; 16:273-82. Epub Mar 15 http://doi.org/10.1177/1740774519833679Google Scholar
- 12.↵Quintó L, Miguel Morales-Asencio, J, González, R, Menéndez, C. Is there sufficient scientific evidence to rule out the use of hydroxychloroquine for postexposure prophylaxis of COVID-19? OSF Preprints 2020. Epub Oct 19 http://doi.org/10.31219/osf.io/d9prqGoogle Scholar
- 13.↵Avidan MS, Dehbi HM, Delany-Moretlwe S. Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med 2020; 383. Epub Jul 15 http://doi.org/10.1056/NEJMc2023617Google Scholar
- 14.↵Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19 – Preliminary Report. N Engl J Med 2020. Epub Jul 17 http://doi.org/10.1056/NEJMoa2021436Google Scholar
- 15.↵Okafor EC, Pastick KA, Rajasingham R. Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. Reply. N Engl J Med 2020; 383. Epub Jul 15 http://doi.org/10.1056/NEJMc2023617Google Scholar
- 16.↵Luco J. Hydroxychloroquine as Post-Exposure Prophylaxis for Covid-19: Why simple data analysis can lead to the wrong conclusions from well-designed studies. ResearchGate 2020. Epub Sep http://doi.org/10.13140/RG.2.2.24214.98880Google Scholar
- 17.↵Watanabe M. Efficacy of Hydroxychloroquine as Prophylaxis for Covid-19. Arxiv 2020:arxiv.org/abs/2007.09477v2. Epub Jul 18Google Scholar
- 18.↵Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial. Ann Intern Med 2020. Epub Jul 16 http://doi.org/10.7326/M20-4207Google Scholar
- 19.↵Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. JBMJ 2013; 346:e7586. Epub 2013/01/11 http://doi.org/10.1136/bmj.e7586Google Scholar
- 20.↵World Health Organization. WHO Trial Registration Data Set (Version 1.3.1). (Accessed August 4, 2020, at https://www.who.int/ictrp/network/trds/en/.)Google Scholar
- 21.↵National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 072420. 2020 July 24. (Accessed 2020 July 27, at https://covid19treatmentguidelines.nih.gov/.)Google Scholar
- 22.↵Ejima K, Kim KS, Ludema C, et al. Estimation of the incubation period of COVID-19 using viral load data. medRxiv 2020:2020.06.16.20132985. Epub June 19 http://doi.org/10.1101/2020.06.16.20132985Google Scholar
- 23.↵Rajasingham R, Bangdiwala AS, Nicol MR, et al. Hydroxychloroquine as pre-exposure prophylaxis for COVID- 19 in healthcare workers: a randomized trial. Clin Infect Dis 2020. Epub Oct 18 http://doi.org/10.1093/cid/ciaa1571Google Scholar
- 24.↵Bhattacharya R, Chowdhury S, Mukherjee R, et al. Pre exposure Hydroxychloroquine use is associated with reduced COVID19 risk in healthcare workers. medRxiv 2020:2020.06.09.20116806. Epub June 12 http://doi.org/10.1101/2020.06.09.20116806Google Scholar
- 25.↵Tan WYT, Wong LY, Leo YS, Toh M. Does incubation period of COVID-19 vary with age? A study of epidemiologically linked cases in Singapore. Epidemiology and infection 2020; 148:e197. Epub Sep 3 http://doi.org/10.1017/S0950268820001995Google Scholar
- 26.↵Guzman-Prado Y. Retraction of Studies on Potential Drug Therapies for COVID-19: A Call for Reliability and Scientific Integrity. Am J Cardiol 2020. Epub Jun 30 http://doi.org/10.1016/j.amjcard.2020.06.061Google Scholar
- 27.↵Karunajeewa H. Hydroxychloroquine for coronavirus: how not to repurpose a drug during a pandemic. Int Med J 2020. Epub Oct 31 http://doi.org/10.1111/imj.15064Google Scholar
- 28.↵Saag MS. Misguided Use of Hydroxychloroquine for COVID-19: The Infusion of Politics Into Science. JAMA 2020. Epub Nov 9 http://doi.org/10.1001/jama.2020.22389Google Scholar
- 29.↵White NJ, Watson JA, Hoglund RM, et al. COVID-19 prevention and treatment: A critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med 2020; 17:e1003252. Epub http://doi.org/10.1371/journal.pmed.1003252CrossRefGoogle Scholar
- 30.↵Horby P, Mafham M, Linsell L, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020. Epub Oct 9 http://doi.org/10.1056/NEJMoa2022926Google Scholar
- 31.↵Frontera JA, Rahimian JO, Yaghi S, et al. Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study. Research square 2020. Epub Nov 4 http://doi.org/10.21203/rs.3.rs-94509/v1Google Scholar
- 32.↵Wiseman DM. Possible synergistic effects of hydroxychloroquine and steroids in COVID-19, time for a nuanced approach. Comment on Arshad et al. Int J Infect Dis 2020; 99:344-5. Epub Aug 10 http://doi.org/10.1016/j.ijid.2020.07.064Google Scholar
- 33.↵Dhibar DDP, Arora DN, Kakkar DA, et al. Post Exposure Prophylaxis with Hydroxychloroquine (HCQ) for the Prevention of COVID-19, a Myth or a Reality? The PEP-CQ Study. Int J Antimicrob Agents 2020:106224. Epub Nov 10 http://doi.org/10.1016/j.ijantimicag.2020.106224Google Scholar
- 34.↵Barnabas R, Brown E, Bershteyn A, et al. LB-17 – Efficacy of Hydroxychloroquine (HCQ) for Post-exposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Blinded, Randomized, Controlled Trial. ID Week 2020. Epub Oct 24Google Scholar
- 35.↵Mitjà O, Corbacho-Monné M, Ubals M, et al. A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19. New England Journal of Medicine 2020. Epub Nov 24 http://doi.org/10.1056/NEJMoa2021801Google Scholar
- 36.↵Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial. JAMA Internal Medicine 2020. Epub Sep 30 http://doi.org/10.1001/jamainternmed.2020.6319Google Scholar
- 37.↵Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007; 357:2189-94. Epub http://doi.org/10.1056/NEJMsr077003CrossRefPubMedWeb of ScienceGoogle Scholar
- 38.↵Cook AJ, Delong E, Murray DM, Vollmer WM, Heagerty PJ. Statistical lessons learned for designing cluster randomized pragmatic clinical trials from the NIH Health Care Systems Collaboratory Biostatistics and Design Core. Clinical trials (London, England) 2016; 13:504-12. Epub May 15 http://doi.org/10.1177/1740774516646578Google Scholar
- 39.↵Reddy KG, Khan MGM, Khan S. Optimum strata boundaries and sample sizes in health surveys using auxiliary variables. PLoS One 2018; 13:e0194787. Epub http://doi.org/10.1371/journal.pone.0194787Google Scholar
- 40.↵Lofgren SM, Nicol MR, Bangdiwala AS, et al. Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19. Open Forum Infect Dis 2020; 7:ofaa500. Epub Nov 19 http://doi.org/10.1093/ofid/ofaa500Google Scholar
Related: Hydroxychloroquine for prophylaxis and treatment of COVID-19 in health care workers: Bulgaria

