Source: hcqmeta.com
• HCQ is effective for COVID-19. The probability that an ineffective treatment generated results as positive as the 235 studies to date is estimated to be 1 in 6 quadrillion (p = 0.00000000000000018).
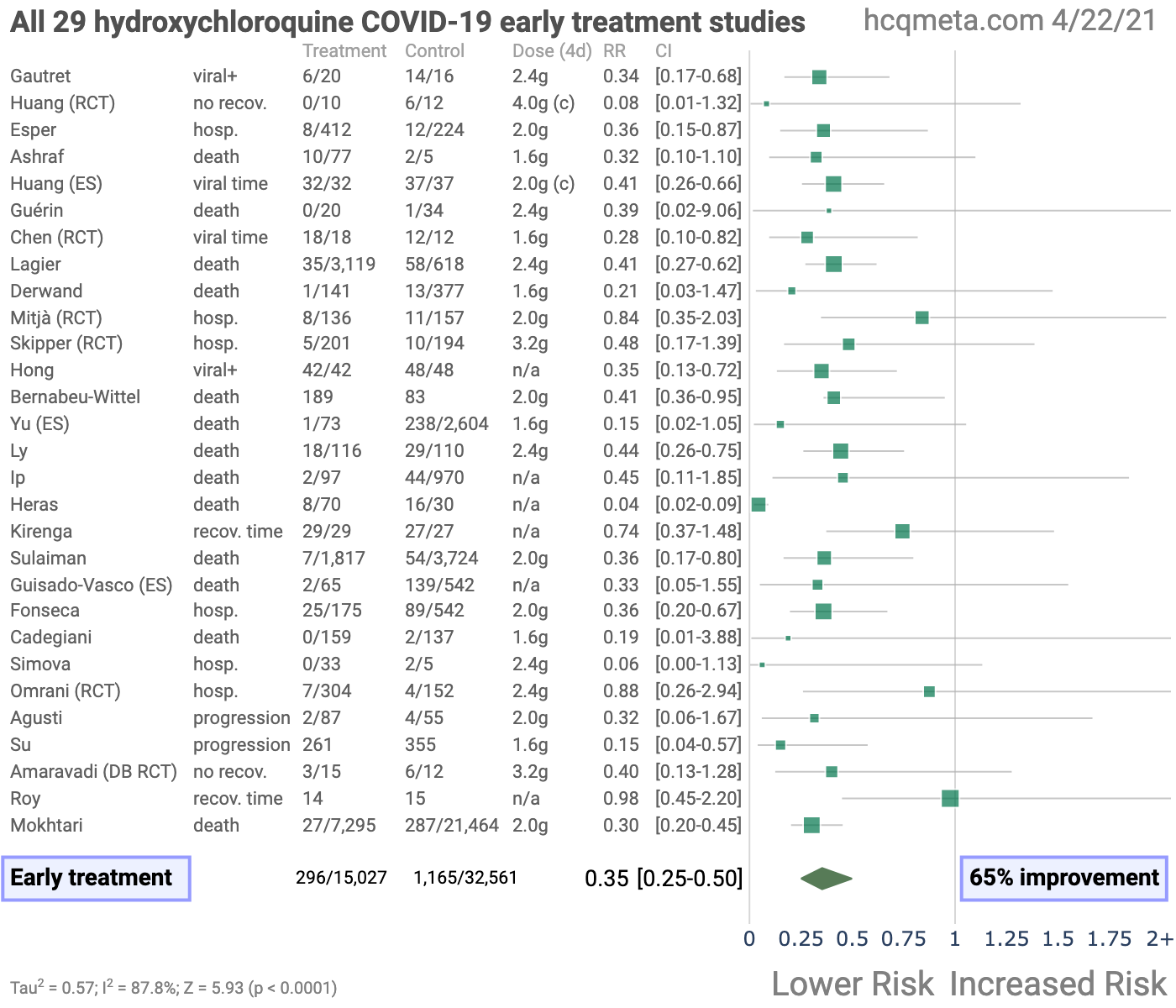
• Early treatment is most successful, with 100% of 29 studies reporting a positive effect (13 statistically significant in isolation) and an estimated reduction of 65% in the effect measured (death, hospitalization, etc.) using a random effects meta-analysis, RR 0.35 [0.25-0.50].
• 92% of Randomized Controlled Trials (RCTs) for early, PrEP, or PEP treatment report positive effects, the probability of this happening for an ineffective treatment is 0.0017.
• There is evidence of bias towards publishing negative results. 88% of prospective studies report positive effects, and only 73% of retrospective studies do.
• Studies from North America are 3.8 times more likely to report negative results than studies from the rest of the world combined, p = 0.0000000015.
• All data to reproduce this paper and the sources are in the appendix.
| Total | 235 studies | 3,740 authors | 359,862 patients |
| Positive effects | 179 studies | 2,743 authors | 251,797 patients |
| Early treatment | 65% improvement | RR 0.35 [0.25-0.50] |
| Late treatment | 23% improvement | RR 0.77 [0.71-0.83] |



Introduction
We analyze all significant studies concerning the use of HCQ (or CQ) for COVID-19. Search methods, inclusion criteria, effect extraction criteria (more serious outcomes have priority), all individual study data, PRISMA answers, and statistical methods are detailed in Appendix 1.
We present random-effects meta-analysis results for all studies, for studies within each treatment stage, for mortality results only, after exclusion of studies with critical bias, and for Randomized Controlled Trials (RCTs) only. Typical meta-analyses involve subjective selection criteria and bias evaluation, requiring an understanding of the criteria and the accuracy of the evaluations.
However, the volume of studies presents an opportunity for an additional simple and transparent analysis aimed at detecting efficacy.
If treatment was not effective, the observed effects would be randomly distributed (or more likely to be negative if treatment is harmful).
We can compute the probability that the observed percentage of positive results (or higher) could occur due to chance with an ineffective treatment (the probability of >= k heads in n coin tosses, or the one-sided sign test / binomial test). Analysis of publication bias is important and adjustments may be needed if there is a bias toward publishing positive results.
For HCQ, we find evidence of a bias toward publishing negative results.
Figure 2 shows stages of possible treatment for COVID-19. Pre-Exposure Prophylaxis (PrEP) refers to regularly taking medication before being infected, in order to prevent or minimize infection. In Post-Exposure Prophylaxis (PEP), medication is taken after exposure but before symptoms appear. Early Treatment refers to treatment immediately or soon after symptoms appear, while Late Treatment refers to more delayed treatment.

Results
Figure 3, Figure 4, and Table 1 show results by treatment stage, and Figure 5 shows a forest plot for a random effects meta-analysis of all studies. Figure 6 shows a forest plot restricted to mortality results only.Early treatment. 100% of early treatment studies report a positive effect, with an estimated reduction of 65% in the effect measured (death, hospitalization, etc.) from the random effects meta-analysis, RR 0.35 [0.25-0.50].
Late treatment.
Late treatment studies are mixed, with 72% showing positive effects, and an estimated reduction of 23% in the random effects meta-analysis. Negative studies mostly fall into the following categories: they show evidence of significant unadjusted confounding, including confounding by indication; usage is extremely late; or they use an excessively high dosage.
Pre-Exposure Prophylaxis.
77% of PrEP studies show positive effects, with an estimated reduction of 29% in the random effects meta-analysis. Negative studies are all studies of systemic autoimmune disease patients which either do not adjust for the different baseline risk of these patients at all, or do not adjust for the highly variable risk within these patients.
Post-Exposure Prophylaxis.
86% of PEP studies report positive effects, with an estimated reduction of 34% in the random effects meta-analysis.
| Treatment time | Number of studies reporting positive results | Total number of studies | Percentage of studies reporting positive results | Probability of an equal or greater percentage of positive results from an ineffective treatment | Random effects meta-analysis results |
| Early treatment | 30 | 30 | 100% | 0.00000000093 1 in 1 billion | 65% improvement RR 0.35 [0.25‑0.50] p < 0.0001 |
| Late treatment | 113 | 158 | 71.5% | 0.000000031 1 in 32 million | 23% improvement RR 0.77 [0.71‑0.83] p < 0.0001 |
| Pre‑Exposure Prophylaxis | 33 | 43 | 76.7% | 0.0003 1 in 3 thousand | 29% improvement RR 0.71 [0.58‑0.87] p = 0.00078 |
| Post‑Exposure Prophylaxis | 6 | 7 | 85.7% | 0.062 1 in 16 | 34% improvement RR 0.66 [0.53‑0.83] p = 0.00043 |
| All studies | 179 | 235 | 76.2% | 0.00000000000000018 1 in 6 quadrillion | 28% improvement RR 0.72 [0.68‑0.77] p < 0.0001 |














Randomized Controlled Trials (RCTs)
Randomized Controlled Trials (RCTs) minimize one source of bias and can provide a higher level of evidence. Results restricted to RCTs are shown in Figure 7, Figure 8, and Table 2. Even with the small number of RCTs to date, they confirm efficacy for early treatment.
While late treatment RCTs are dominated by the very late stage and large RECOVERY/SOLIDARITY trials, prophylaxis and early treatment studies show 32% improvement in random effects meta-analysis, RR 0.68 [0.54‑0.85], p = 0.00068. Early treatment RCTs show 49% improvement, RR 0.51 [0.32‑0.82], p = 0.005.Evidence supports incorporating non-RCT studies. [Concato] find that well-designed observational studies do not systematically overestimate the magnitude of the effects of treatment compared to RCTs.
[Anglemyer] summarized reviews comparing RCTs to observational studies and found little evidence for significant differences in effect estimates.
[Lee] shows that only 14% of the guidelines of the Infectious Diseases Society of America were based on RCTs. Limitations in an RCT can easily outweigh the benefits, for example excessive dosages, excessive treatment delays, or Internet survey bias could easily have a greater effect on results. Ethical issues may prevent running RCTs for known effective treatments. For more on the problems with RCTs see [Deaton, Nichol].






| Treatment time | Number of studies reporting positive results | Total number of studies | Percentage of studies reporting positive results | Probability of an equal or greater percentage of positive results from an ineffective treatment | Random effects meta-analysis results |
| Randomized Controlled Trials | 24 | 30 | 80.0% | 0.00072 1 in 1 thousand | 29% improvement RR 0.71 [0.57‑0.89] p = 0.0029 |
| Randomized Controlled Trials (excluding late treatment) | 12 | 13 | 92.3% | 0.0017 1 in 585 | 32% improvement RR 0.68 [0.54‑0.85] p = 0.00068 |
Analysis with ExclusionsMany meta-analyses for HCQ have been written, most of which have become somewhat obselete due to the continuing stream of more recent studies. Recent analyses with positive conclusions include [IHU Marseille] which considers significant bias from an understanding of each trial, and [Garcia-Albeniz, Ladapo, Prodromos] which focus on early or prophylactic use studies.Meta analyses reporting negative conclusions focus on late treatment studies, tend to disregard treatment delay, tend to follow formulaic evaluations which overlook major issues with various studies, and end up with weighting disproportionate to a reasoned analysis of each study’s contribution. For example, [Axfors] assigns 87% weight to a single trial, the RECOVERY trial [RECOVERY], thereby producing the same result. However, the RECOVERY trial may be the most biased of the studies they included, due to the excessive dosage used, close to the level shown to be very dangerous in [Borba] (OR 2.8), and with extremely sick late stage patients (60% requiring oxygen, 17% ventilation/ECMO, and a very high mortality rate in both arms). There is little reason to suggest that the results from this trial are applicable to more typical dosages or to earlier treatment (10/22: the second version of this study released 10/22 assigns 74% to RECOVERY and 15% to SOLIDARITY [SOLIDARITY], which is the only other trial using a similar excessive dosage).We include all studies in the main analysis, however there are major issues with several studies that could significantly alter the results.
Here, we present an analysis excluding studies with significant issues, including indication of significant unadjusted group differences or confouding by indication, extremely late stage usage >14 days post symptoms or >50% on oxygen at baseline, very minimal detail provided, excessive dosages which have been shown to be dangerous, significant issues with adjustments that could reasonably make substantial differences, and reliance on PCR which may be inaccurate and less indicative of severity than symptoms.
The aim here is not to exclude studies on technicalities, but to exclude studies that clearly have major issues that may significantly change the outcome. We welcome feedback on improvements or corrections to this. The studies excluded are as follows, and the resulting forest plot is shown in Figure 9.
[Ader], very late stage, >50% on oxygen/ventilation at baseline.
[Alamdari], substantial unadjusted confounding by indication likely.
[Albani], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically.
[Alghamdi], confounding by indication is likely and adjustments do not consider COVID-19 severity.
[An], results only for PCR status which may be significantly different to symptoms.
[Annie], confounding by indication is likely and adjustments do not consider COVID-19 severity.
[Awad], substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, substantial unadjusted confounding by indication likely.
[Barbosa], excessive unadjusted differences between groups.
[Budhiraja], excessive unadjusted differences between groups.
[Cassione], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Chen], results only for PCR status which may be significantly different to symptoms.
[Chen (B)], results only for PCR status which may be significantly different to symptoms.
[Chen (C)], results only for PCR status which may be significantly different to symptoms.
[Choi], excessive unadjusted differences between groups.
[Cravedi], substantial unadjusted confounding by indication likely.
[de la Iglesia], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Fitzgerald], not fully adjusting for the baseline risk differences within systemic autoimmune patients.
[Fried], excessive unadjusted differences between groups, substantial unadjusted confounding by indication likely.
[Gautret], excessive unadjusted differences between groups, results only for PCR status which may be significantly different to symptoms.
[Geleris], significant issues found with adjustments.
[Gendebien], not fully adjusting for the baseline risk differences within systemic autoimmune patients.
[Gendelman], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Gianfrancesco], not fully adjusting for the baseline risk differences within systemic autoimmune patients.
[Gupta], very late stage, >50% on oxygen/ventilation at baseline.
[Hong], results only for PCR status which may be significantly different to symptoms.
[Hraiech], very late stage, ICU patients.
[Huang], significant unadjusted confounding possible.
[Huang (B)], results only for PCR status which may be significantly different to symptoms.
[Huang (C)], results only for PCR status which may be significantly different to symptoms.
[Huh], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Huh (B)], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Izoulet], excessive unadjusted differences between groups.
[Kamran], excessive unadjusted differences between groups.
[Kelly], substantial unadjusted confounding by indication likely.
[Konig], not fully adjusting for the baseline risk differences within systemic autoimmune patients.
[Kuderer], substantial unadjusted confounding by indication likely.
[Lamback], substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically.
[Laplana], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Lecronier], very late stage, >50% on oxygen/ventilation at baseline.
[Lotfy], substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, substantial unadjusted confounding by indication likely.
[Luo], substantial unadjusted confounding by indication likely.
[Lyngbakken], results only for PCR status which may be significantly different to symptoms.
[Macias], not fully adjusting for the baseline risk differences within systemic autoimmune patients.
[McGrail], excessive unadjusted differences between groups.
[Mitchell], excessive unadjusted differences between groups.
[Peters], excessive unadjusted differences between groups.
[Psevdos], unadjusted results with no group details, no treatment details, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, substantial unadjusted confounding by indication likely.
[Rangel], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[RECOVERY], excessive dosage, results do not apply to typical dosages.
[Rentsch], not fully adjusting for the baseline risk differences within systemic autoimmune patients, medication adherence unknown and may significantly change results.
[Rodriguez-Nava], substantial unadjusted confounding by indication likely, excessive unadjusted differences between groups.[Roomi], substantial unadjusted confounding by indication likely.
[Roy], no serious outcomes reported and fast recovery in treatment and control groups, there is little room for a treatment to improve results.
[Salazar], substantial unadjusted confounding by indication likely.
[Saleemi], results only for PCR status which may be significantly different to symptoms, substantial unadjusted confounding by indication likely.
[Salvarani], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Sands], includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons, substantial unadjusted confounding by indication likely.
[Sarfaraz], substantial unadjusted confounding by indication likely, significant unadjusted confounding possible.
[Sbidian], significant issues found with adjustments.
[Shabrawishi], results only for PCR status which may be significantly different to symptoms.
[Singer], not fully adjusting for the baseline risk differences within systemic autoimmune patients.
[Singh], confounding by indication is likely and adjustments do not consider COVID-19 severity.
[Solh], very late stage, >50% on oxygen/ventilation at baseline, substantial unadjusted confounding by indication likely.
[SOLIDARITY], excessive dosage, results do not apply to typical dosages, very late stage, >50% on oxygen/ventilation at baseline.
[Sosa-García], very late stage, >50% on oxygen/ventilation at baseline, substantial unadjusted confounding by indication likely.
[Soto-Becerra], substantial unadjusted confounding by indication likely, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart (B)], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart (C)], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart (D)], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart (E)], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart (F)], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Stewart (G)], substantial unadjusted confounding by indication likely, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Tang], results only for PCR status which may be significantly different to symptoms.
[Tehrani], substantial unadjusted confounding by indication likely.
[Texeira], unadjusted results with no group details, no treatment details, substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, substantial unadjusted confounding by indication likely.
[Trefond], not fully adjusting for the different baseline risk of systemic autoimmune patients, significant unadjusted confounding possible, excessive unadjusted differences between groups.
[Ubaldo], substantial unadjusted confounding by indication likely, very late stage, ICU patients.
[Ulrich], very late stage, >50% on oxygen/ventilation at baseline.
[Vernaz], substantial time varying confounding likely due to declining usage over the early period when overall treatment protocols improved dramatically, substantial unadjusted confounding by indication likely.
[Vivanco-Hidalgo], not fully adjusting for the different baseline risk of systemic autoimmune patients.
[Wang], confounding by indication is likely and adjustments do not consider COVID-19 severity.
[Xia], detail too minimal.
[Yegerov], unadjusted results with no group details.
[Zhong], results only for PCR status which may be significantly different to symptoms.



Discussion
Publication bias.
Publishing is often biased towards positive results, which we would need to adjust for when analyzing the percentage of positive results. Studies that require less effort are considered to be more susceptible to publication bias. Prospective trials that involve significant effort are likely to be published regardless of the result, while retrospective studies are more likely to exhibit bias.
For example, researchers may perform preliminary analysis with minimal effort and the results may influence their decision to continue. Retrospective studies also provide more opportunities for the specifics of data extraction and adjustments to influence results.
For HCQ, 87.8% of prospective studies report positive effects, compared to 73.1% of retrospective studies, indicating a bias toward publishing negative results.
Figure 10 shows a scatter plot of results for prospective and retrospective studies.
Figure 11 shows the results by region of the world, for all regions that have > 5 studies. Studies from North America are 3.8 times more likely to report negative results than studies from the rest of the world combined, 53.4% vs. 14.1%, two-tailed z test -6.05, p = 0.0000000015.
[Berry] performed an independent analysis which also showed bias toward negative results for US-based research.


The lack of bias towards positive results is not very surprising. Both negative and positive results are very important given the current use of HCQ for COVID-19 around the world, evidence of which can be found in the studies analyzed here, government protocols, and news reports, for example [AFP, AfricaFeeds, Africanews, Afrik.com, Al Arabia, Al-bab, Anadolu Agency, Anadolu Agency (B), Archyde, Barron’s, Barron’s (B), BBC, Belayneh, A., Bianet, CBS News, Challenge, Dr. Goldin, Efecto Cocuyo, Expats.cz, Face 2 Face Africa, Filipova, France 24, France 24 (B), Franceinfo, Global Times, Government of China, Government of India, Government of Venezuela, GulfInsider, Le Nouvel Afrik, LifeSiteNews, Medical World Nigeria, Medical Xpress, Medical Xpress (B), Middle East Eye, Ministerstva Zdravotnictví, Ministry of Health of Ukraine, Ministry of Health of Ukraine (B), Morocco World News, Mosaique Guinee, Nigeria News World, NPR News, Oneindia, Pan African Medical Journal, Parola, Pilot News, PledgeTimes, Pleno.News, Q Costa Rica, Rathi, Russian Government, Russian Government (B), Teller Report, The Africa Report, The Australian, The BL, The East African, The Guardian, The Indian Express, The Moscow Times, The North Africa Post, The Tico Times, Ukrinform, Vanguard, Voice of America].We also note a bias towards publishing negative results by certain journals and press organizations, with scientists reporting difficulty publishing positive results [Boulware, Meneguesso].
Although 179 studies show positive results, The New York Times, for example, has only written articles for studies that claim HCQ is not effective [The New York Times, The New York Times (B), The New York Times (C)]. As of September 10, 2020, The New York Times still claims that there is clear evidence that HCQ is not effective for COVID-19 [The New York Times (D)]. As of October 9, 2020, the United States National Institutes of Health recommends against HCQ for both hospitalized and non-hospitalized patients [United States National Institutes of Health].
Treatment details.
We focus here on the question of whether HCQ is effective or not for COVID-19. Studies vary significantly in terms of treatment delay, treatment regimen, patients characteristics, and (for the pooled effects analysis) outcomes, as reflected in the high degree of heterogeneity. However, early treatment consistently shows benefits. 100% of early treatment studies report a positive effect, with an estimated reduction of 65% in the effect measured (death, hospitalization, etc.) in the random effects meta-analysis, RR 0.35 [0.25-0.50].
Conclusion
HCQ is an effective treatment for COVID-19. The probability that an ineffective treatment generated results as positive as the 235 studies to date is estimated to be 1 in 6 quadrillion (p = 0.00000000000000018).
100% of early treatment studies report a positive effect, with an estimated reduction of 65% in the effect measured (death, hospitalization, etc.) using a random effects meta-analysis, RR 0.35 [0.25-0.50].
Revisions
This paper is data driven, all graphs and numbers are dynamically generated. We will update the paper as new studies are released or with any corrections. Please submit updates and corrections at the bottom of this page.
10/21: We added studies [Dubee, Martinez-Lopez, Solh]. We received a report that the United States National Institutes of Health is recommending against HCQ for hospitalized and non-hospitalized patients as of October 9, and we added a reference.
10/22: We added [Anglemyer, Ñamendys-Silva]. We updated the discussion of [Axfors] for the second version of this study. We added a table summarizing RCT results.
10/23: We added [Komissarov, Lano]. The second version of the preprint for [Komissarov] includes a comparison with the control group (not reported in the first version). We updated [Lyngbakken] to use the mortality result in the recent journal version of the paper (not reported in the preprint).
10/26: We added [Coll, Goenka, Synolaki].
10/28: We added [Arleo, Choi].10/30: We added [Berenguer, Faíco-Filho].
10/31: We added [Fonseca, Frontera, Tehrani].
11/1: We added [Trullàs].
11/4: We added [Behera, Cadegiani].11/8: We added [Dhibar].
11/9: We added [Self].
11/10: We added [Mathai].
11/12: We added [Simova, Simova (B)].
11/13: We added [Núñez-Gil, Águila-Gordo].
11/14: We added [Sheshah].
11/18: We added [Budhiraja].
11/19: We added [Falcone].
11/20: We added [Omrani].
11/23: We added [Revollo].
11/24: We added [Boari].
11/25: We added [Qin], and we added analysis restricted to mortality results.
11/27: We added [van Halem].
11/28: We added [Lambermont].
11/30: We added [Abdulrahman].
12/1: We added [Capsoni].
12/2: We added [Rodriguez-Gonzalez].
12/4: We added [Modrák, Ozturk, Peng].
12/7: We added [Maldonado].
12/8: We added [Barnabas].
12/9: We added [Agusti, Guglielmetti].
12/11: We added [Jung].
12/13: We added [Bielza].
12/14: We added [Rivera-Izquierdo, Rodriguez-Nava].
12/15: We added [Kalligeros, López].
12/16: We added [Alqassieh, Naseem, Orioli, Sosa-García, Tan].
12/17: We added [Signes-Costa].
12/20: We added [Gönenli, Huh].
12/21: We added [Matangila].
12/22: We added [Taccone].
12/23: We added [Cangiano].
12/24: We added [Su].
12/25: We added [Chari].
12/27: We added the total number of authors and patients.
12/28: We added [Auld, Cordtz].
12/29: We added [Güner, Salazar].
12/31: We added additional details about the studies in the appendix.
1/1: We added [Sands].
1/2: We added the number of patients to the forest plots.
1/3: We added dosage information for early treatment studies.
1/4: We added [Vernaz].
1/5: We added [Sarfaraz].
1/6: We added direct links to the study details in the forest plots.
1/7: We added direct links to the study details in the chronological plots.
1/9: We added [Texeira, Yegerov].
1/11: We added [Rangel].
1/12: We added [Li (B)].
1/15: We updated [Ip] to the published version.
1/16: We added the effect measured for each study in the forest plots.
1/21: We added [Li].
1/24: We added [Desbois, Psevdos]. We moved the analysis with exclusions and mortality analysis to the main text.
2/1: We added [Trefond].
2/2: We added [Bernabeu-Wittel].
2/5: We added [Hernandez-Cardenas].
2/6: We added [Fitzgerald].
2/7: We added [Johnston].
2/9: We added [Ouedraogo].
2/10: We added [Roig, Ubaldo].
2/15: We added [Lora-Tamayo].
2/16: We added [Albani].
2/17: We added [Purwati].
2/18: We added [Awad].
2/20: We added [Lamback].
2/23: We added [Beltran-Gonzalez (B)].
2/25: We added [Bae].
2/26: We added [Amaravadi].
2/28: We added [Rodriguez].
3/2: We added [Pham].
3/3: We added [Pasquini].
3/5: We added [Lotfy].
3/7: We added [Salvador].
3/8: We added [Martin-Vicente].
3/9: We added [Vivanco-Hidalgo].
3/13: We added [Roy].
3/24: We added [Dev].
3/27: We added [Hraiech], and we corrected an error in effect extraction for [Self].
3/28: We added [Stewart].
3/29: We added [Barry].
4/1: We added [Alghamdi].
4/2: We added [Salvarani].
4/4: We updated [Mitjà] for 11 control hospitalizations. There is conflicting data, table S2 lists 12 control hospitalizations, while table 2 shows 11. A previous version of this paper also showed some values corresponding to 12 control hospitalizations in the abstract and table 2.
4/6: We added [Mokhtari].
4/9: We updated [Dubee] to the journal version
.4/14: We added [Seet].
4/20: We added [Alegiani, Alzahrani].
Appendix 1. Methods and Study Results
We performed ongoing searches of PubMed, medRxiv, ClinicalTrials.gov, The Cochrane Library, Google Scholar, Collabovid, Research Square, ScienceDirect, Oxford University Press, the reference lists of other studies and meta-analyses, and submissions to the site c19hcq.com, which regularly receives submissions of both positive and negative studies upon publication.
Search terms were hydroxychloroquine or chloroquine and COVID-19 or SARS-CoV-2, or simply hydroxychloroquine or chloroquine. Automated searches are performed every hour with notifications of new matches.
All studies regarding the use of HCQ or CQ for COVID-19 that report an effect compared to a control group are included in the main analysis. This is a living analysis and is updated regularly.We extracted effect sizes and associated data from all studies. If studies report multiple kinds of effects then the most serious outcome is used in calculations for that study. For example, if effects for mortality and cases are both reported, the effect for mortality is used, this may be different to the effect that a study focused on.
If symptomatic results are reported at multiple times, we used the latest time, for example if mortality results are provided at 14 days and 28 days, the results at 28 days are used. Mortality alone is preferred over combined outcomes. Outcomes with zero events in both arms were not used. Clinical outcome is considered more important than PCR testing status.
When basically all patients recover in both treatment and control groups, preference for viral clearance and recovery is given to results mid-recovery where available (after most or all patients have recovered there is no room for an effective treatment to do better). When results provide an odds ratio, we computed the relative risk when possible, or converted to a relative risk according to [Zhang]. Reported confidence intervals and p-values were used when available, using adjusted values when provided. If multiple types of adjustments are reported including propensity score matching (PSM), the PSM results are used.
When needed, conversion between reported p-values and confidence intervals followed [Altman, Altman (B)], and Fisher’s exact test was used to calculate p-values for event data. If continuity correction for zero values is required, we use the reciprocal of the opposite arm with the sum of the correction factors equal to 1 [Sweeting].
If a study separates HCQ and HCQ+AZ, we use the combined results were possible, or the results for the larger group. Results are all expressed with RR < 1.0 suggesting effectiveness. Most results are the relative risk of something negative. If a study reports relative times, the results are expressed as the ratio of the time for the HCQ group versus the time for the control group.
If a study reports the rate of reduction of viral load, the results are based on the percentage change in the rate. Calculations are done in Python (3.9.1) with scipy (1.5.4), pythonmeta (1.11), numpy (1.19.4), statsmodels (0.12.1), and plotly (4.14.1).The forest plots are computed using PythonMeta [Deng] with the DerSimonian and Laird random effects model (the fixed effect assumption is not plausible in this case).
We received no funding, this research is done in our spare time. We have no affiliations with any pharmaceutical companies or political parties.
We have classified studies as early treatment if most patients are not already at a severe stage at the time of treatment, and treatment started within 5 days after the onset of symptoms, although a shorter time may be preferable. Antivirals are typically only considered effective when used within a shorter timeframe, for example 0-36 or 0-48 hours for oseltamivir, with longer delays not being effective [McLean, Treanor].
A summary of study results is below. Please submit updates and corrections at the bottom of this page.
Early treatment
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| Agusti], 12/9/2020, prospective, Spain, Europe, peer-reviewed, median age 37.0, 13 authors, dosage 400mg bid day 1, 200mg bid days 2-5. | risk of disease progression, 68.4% lower, RR 0.32, p = 0.21, treatment 2 of 87 (2.3%), control 4 of 55 (7.3%), pneumonia. |
| time to viral-, 31.8% lower, relative time 0.68, treatment 87, control 55. | |
| [Amaravadi], 2/26/2021, Double Blind Randomized Controlled Trial, USA, North America, preprint, 20 authors, dosage 400mg bid days 1-14. | risk of not reaching lowest symptom score at day 7 mid-recovery, 60.0% lower, RR 0.40, p = 0.13, treatment 3 of 15 (20.0%), control 6 of 12 (50.0%). |
| relative time to first occurrence of lowest symptom score, 42.9% lower, relative time 0.57, p = 0.21, treatment 15, control 12. | |
| relative time to release from quarantine, 27.3% lower, relative time 0.73, p = 0.28, treatment 16, control 13. | |
| [Ashraf], 4/24/2020, retrospective, database analysis, Iran, Middle East, preprint, median age 58.0, 16 authors, dosage 200mg bid daily, 400mg qd was used when combined with Lopinavir-Ritonavir. | risk of death, 67.5% lower, RR 0.32, p = 0.15, treatment 10 of 77 (13.0%), control 2 of 5 (40.0%). |
| [Bernabeu-Wittel], 8/1/2020, retrospective, Spain, Europe, peer-reviewed, 13 authors, dosage 400mg bid day 1, 200mg bid days 2-7. | risk of death, 59.0% lower, RR 0.41, p = 0.03, treatment 189, control 83. |
| [Cadegiani], 11/4/2020, prospective, Brazil, South America, preprint, 4 authors, dosage 400mg days 1-5. | risk of death, 81.2% lower, RR 0.19, p = 0.21, treatment 0 of 159 (0.0%), control 2 of 137 (1.5%), continuity correction due to zero event (with reciprocal of the contrasting arm), control group 1. |
| risk of mechanical ventilation, 95.1% lower, RR 0.05, p < 0.001, treatment 0 of 159 (0.0%), control 9 of 137 (6.6%), continuity correction due to zero event (with reciprocal of the contrasting arm), control group 1. | |
| risk of hospitalization, 98.3% lower, RR 0.02, p < 0.001, treatment 0 of 159 (0.0%), control 27 of 137 (19.7%), continuity correction due to zero event (with reciprocal of the contrasting arm), control group 1. | |
| [Chen], 6/22/2020, Randomized Controlled Trial, China, Asia, preprint, 19 authors, dosage 200mg bid days 1-10. | median time to PCR-, 72.0% lower, relative time 0.28, p = 0.01, treatment 18, control 12. |
| [Derwand], 10/26/2020, retrospective, USA, North America, peer-reviewed, 3 authors, dosage 200mg bid days 1-5. | risk of death, 79.4% lower, RR 0.21, p = 0.12, treatment 1 of 141 (0.7%), control 13 of 377 (3.4%), odds ratio converted to relative risk. |
| risk of hospitalization, 81.6% lower, RR 0.18, p < 0.001, treatment 4 of 141 (2.8%), control 58 of 377 (15.4%), odds ratio converted to relative risk. | |
| [Esper], 4/15/2020, prospective, Brazil, South America, preprint, 15 authors, dosage 800mg day 1, 400mg days 2-7. | risk of hospitalization, 64.0% lower, RR 0.36, p = 0.02, treatment 8 of 412 (1.9%), control 12 of 224 (5.4%). |
| [Fonseca], 10/31/2020, retrospective, Brazil, South America, peer-reviewed, mean age 50.6, 10 authors, dosage 400mg bid day 1, 400mg qd days 2-5. | risk of hospitalization, 64.0% lower, RR 0.36, p < 0.001, treatment 25 of 175 (14.3%), control 89 of 542 (16.4%), adjusted per study, odds ratio converted to relative risk, HCQ vs. nothing. |
| risk of hospitalization, 50.5% lower, RR 0.49, p = 0.006, treatment 25 of 175 (14.3%), control 89 of 542 (16.4%), adjusted per study, odds ratio converted to relative risk, HCQ vs. anything else. | |
| [Gautret], 3/17/2020, prospective, France, Europe, peer-reviewed, 18 authors, dosage 200mg tid days 1-10. | risk of no virological cure at day 6, 66.0% lower, RR 0.34, p = 0.001, treatment 6 of 20 (30.0%), control 14 of 16 (87.5%). |
| [Guisado-Vasco], 10/15/2020, retrospective, Spain, Europe, peer-reviewed, median age 69.0, 25 authors, early treatment subset, dosage not specified. | risk of death, 66.9% lower, RR 0.33, p = 0.19, treatment 2 of 65 (3.1%), control 139 of 542 (25.6%), adjusted per study, odds ratio converted to relative risk, multivariate. |
| [Guérin], 5/31/2020, retrospective, France, Europe, peer-reviewed, 8 authors, dosage 600mg days 1-10, 7-10 days. | risk of death, 61.4% lower, RR 0.39, p = 1.00, treatment 0 of 20 (0.0%), control 1 of 34 (2.9%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| recovery time, 65.0% lower, relative time 0.35, p < 0.001, treatment 20, control 34. | |
| [Heras], 9/2/2020, retrospective, Andorra, Europe, peer-reviewed, median age 85.0, 13 authors, dosage not specified. | risk of death, 95.6% lower, RR 0.04, p = 0.004, treatment 8 of 70 (11.4%), control 16 of 30 (53.3%), adjusted per study. |
| [Hong], 7/16/2020, retrospective, South Korea, Asia, peer-reviewed, 7 authors, dosage not specified. | risk of prolonged viral shedding, 64.9% lower, RR 0.35, p = 0.001, treatment 42, control 48, odds ratio converted to relative risk. |
| [Huang (B)], 5/28/2020, prospective, China, Asia, peer-reviewed, 36 authors, early treatment subset, dosage chloroquine 500mg days 1-10, two groups, 500mg qd and 500mg bid. | time to viral-, 59.1% lower, relative time 0.41, p < 0.001, treatment 32, control 37. |
| [Huang (D)], 4/1/2020, Randomized Controlled Trial, China, Asia, peer-reviewed, 18 authors, dosage chloroquine 500mg bid days 1-10. | risk of no recovery at day 14, 91.7% lower, RR 0.08, p = 0.02, treatment 0 of 10 (0.0%), control 6 of 12 (50.0%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| risk of no improvement in pneumonia at day 14, 83.0% lower, RR 0.17, p = 0.22, treatment 10, control 12. | |
| [Ip], 8/25/2020, retrospective, database analysis, USA, North America, peer-reviewed, 25 authors, dosage not specified. | risk of death, 54.5% lower, RR 0.45, p = 0.43, treatment 2 of 97 (2.1%), control 44 of 970 (4.5%). |
| risk of ICU admission, 28.6% lower, RR 0.71, p = 0.79, treatment 3 of 97 (3.1%), control 42 of 970 (4.3%). | |
| risk of hospitalization, 37.3% lower, RR 0.63, p = 0.04, treatment 21 of 97 (21.6%), control 305 of 970 (31.4%), adjusted per study, odds ratio converted to relative risk. | |
| [Izoulet], 4/21/2020, retrospective, multiple countries, multiple regions, preprint, 1 author, dosage not specified. | risk of death, 85.0% lower, RR 0.15, p < 0.001. |
| [Kirenga], 9/9/2020, prospective, Uganda, Africa, peer-reviewed, 29 authors, dosage not specified. | median time to recovery, 25.6% lower, relative time 0.74, p = 0.20, treatment 29, control 27. |
| [Lagier], 6/25/2020, retrospective, France, Europe, peer-reviewed, 22 authors, dosage 200mg tid days 1-10. | risk of death, 59.0% lower, RR 0.41, p = 0.05, treatment 35 of 3119 (1.1%), control 58 of 618 (9.4%), adjusted per study. |
| [Ly], 8/21/2020, retrospective, France, Europe, peer-reviewed, mean age 83.0, 21 authors, dosage 200mg tid days 1-10. | risk of death, 55.6% lower, RR 0.44, p = 0.02, treatment 18 of 116 (15.5%), control 29 of 110 (26.4%), adjusted per study, odds ratio converted to relative risk. |
| [Mitjà], 7/16/2020, Randomized Controlled Trial, Spain, Europe, peer-reviewed, 45 authors, dosage 800mg day 1, 400mg days 2-7. | risk of hospitalization, 16.0% lower, RR 0.84, p = 0.64, treatment 8 of 136 (5.9%), control 11 of 157 (7.0%). |
| risk of no recovery, 34.0% lower, RR 0.66, p = 0.38, treatment 8 of 136 (5.9%), control 14 of 157 (8.9%). | |
| elative change in viral load from baseline, 2.0% lower, relative load 0.98, treatment 136, control 157, day 7. | |
| [Mokhtari], 4/6/2021, retrospective, Iran, Middle East, peer-reviewed, 11 authors, dosage 400mg bid day 1, 200mg bid days 2-5. | risk of death, 69.7% lower, RR 0.30, p < 0.001, treatment 27 of 7295 (0.4%), control 287 of 21464 (1.3%), adjusted per study, odds ratio converted to relative risk. |
| risk of hospitalization, 35.3% lower, RR 0.65, p < 0.001, treatment 523 of 7295 (7.2%), control 2382 of 21464 (11.1%), adjusted per study, odds ratio converted to relative risk. | |
| [Omrani], 11/20/2020, Randomized Controlled Trial, Qatar, Middle East, peer-reviewed, 19 authors, dosage 600mg days 1-6. | risk of hospitalization, 12.5% lower, RR 0.88, p = 1.00, treatment 7 of 304 (2.3%), control 4 of 152 (2.6%), HCQ+AZ or HCQ vs. control. |
| risk of symptomatic at day 21, 25.8% lower, RR 0.74, p = 0.58, treatment 9 of 293 (3.1%), control 6 of 145 (4.1%), HCQ+AZ or HCQ vs. control. | |
| risk of Ct<=40 at day 14, 10.3% higher, RR 1.10, p = 0.13, treatment 223 of 295 (75.6%), control 98 of 143 (68.5%), HCQ+AZ or HCQ vs. control. | |
| [Roy], 3/12/2021, retrospective, database analysis, India, South Asia, preprint, 5 authors, dosage not specified. | relative time to clinical response of wellbeing, 2.4% lower, relative time 0.98, p = 0.96, treatment 14, control 15. |
| [Simova], 11/12/2020, retrospective, Bulgaria, Europe, peer-reviewed, 5 authors, dosage 200mg tid days 1-14. | risk of hospitalization, 93.8% lower, RR 0.06, p = 0.01, treatment 0 of 33 (0.0%), control 2 of 5 (40.0%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| risk of viral+ at day 14, 95.8% lower, RR 0.04, p = 0.001, treatment 0 of 33 (0.0%), control 3 of 5 (60.0%), continuity correction due to zero event (with reciprocal of the contrasting arm). | |
| [Skipper], 7/16/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 24 authors, dosage 800mg once, followed by 600mg in 6 to 8 hours, then 600mg daily for 4 more days. | risk of hospitalization, 51.7% lower, RR 0.48, p = 0.19, treatment 5 of 201 (2.5%), control 10 of 194 (5.2%). |
| risk of no recovery at day 14, 20.0% lower, RR 0.80, p = 0.21. | |
| [Su], 12/23/2020, retrospective, China, Asia, peer-reviewed, 9 authors, dosage 400mg days 1-10, 400mg daily for 10-14 days. | risk of disease progression, 84.9% lower, RR 0.15, p = 0.006, treatment 261, control 355, adjusted per study, binary logistic regression. |
| improvement time, 24.0% lower, relative time 0.76, p = 0.02, treatment 261, control 355, adjusted per study, Cox proportional hazards regression. | |
| [Sulaiman], 9/13/2020, prospective, Saudi Arabia, Middle East, preprint, 22 authors, dosage 400mg bid day 1, 200mg bid days 2-5. | risk of death, 63.7% lower, RR 0.36, p = 0.01, treatment 7 of 1817 (0.4%), control 54 of 3724 (1.5%), adjusted per study, odds ratio converted to relative risk. |
| risk of hospitalization, 38.6% lower, RR 0.61, p = 0.001, treatment 171 of 1817 (9.4%), control 617 of 3724 (16.6%), adjusted per study, odds ratio converted to relative risk. | |
| [Yu], 8/3/2020, retrospective, China, Asia, preprint, median age 62.0, 6 authors, early treatment subset, dosage 200mg bid days 1-10. | risk of death, 85.0% lower, RR 0.15, p = 0.02, treatment 1 of 73 (1.4%), control 238 of 2604 (9.1%), HCQ treatment started early vs. non-HCQ. |
Late treatment
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| [Abd-Elsalam], 8/14/2020, Randomized Controlled Trial, Egypt, Africa, peer-reviewed, 10 authors. | risk of death, 20.0% higher, RR 1.20, p = 1.00, treatment 6 of 97 (6.2%), control 5 of 97 (5.2%). |
| risk of no recovery at day 28, 30.0% lower, RR 0.70, p = 0.009, treatment 45 of 97 (46.4%), control 64 of 97 (66.0%). | |
| [Abdulrahman], 11/30/2020, retrospective, Bahrain, Middle East, preprint, 9 authors. | risk of death, 16.7% lower, RR 0.83, p = 1.00, treatment 5 of 223 (2.2%), control 6 of 223 (2.7%), PSM. |
| risk of combined intubation/death, 75.0% higher, RR 1.75, p = 0.24, treatment 12 of 223 (5.4%), control 7 of 223 (3.1%), adjusted per study, PSM. | |
| [Ader], 10/6/2020, Randomized Controlled Trial, multiple countries, Europe, preprint, baseline oxygen requirements 95.4%, 52 authors. | risk of death at day 29, 6.4% lower, RR 0.94, p = 1.00, treatment 11 of 145 (7.6%), control 12 of 148 (8.1%). |
| [Alamdari], 9/9/2020, retrospective, Iran, West Asia, peer-reviewed, 14 authors. | risk of death, 55.0% lower, RR 0.45, p = 0.03, treatment 427, control 32. |
| [Albani], 8/30/2020, retrospective, Italy, Europe, peer-reviewed, 11 authors. | risk of death, 18.4% lower, RR 0.82, p = 0.05, treatment 60 of 211 (28.4%), control 172 of 605 (28.4%), adjusted per study, odds ratio converted to relative risk, HCQ vs. neither. |
| risk of death, 9.0% higher, RR 1.09, p = 0.38, treatment 60 of 211 (28.4%), control 172 of 605 (28.4%), adjusted per study, odds ratio converted to relative risk, HCQ+AZ vs. neither. | |
| risk of ICU admission, 9.2% higher, RR 1.09, p = 0.68, treatment 73 of 211 (34.6%), control 46 of 605 (7.6%), adjusted per study, odds ratio converted to relative risk, HCQ vs. neither. | |
| risk of ICU admission, 71.3% higher, RR 1.71, p < 0.001, treatment 73 of 211 (34.6%), control 46 of 605 (7.6%), adjusted per study, odds ratio converted to relative risk, HCQ+AZ vs. neither. | |
| [Alberici], 5/10/2020, retrospective, Italy, Europe, peer-reviewed, 31 authors. | risk of death, 42.9% lower, RR 0.57, p = 0.12, treatment 17 of 72 (23.6%), control 9 of 22 (40.9%), odds ratio converted to relative risk. |
| [Alghamdi], 3/31/2021, retrospective, Saudi Arabia, Middle East, peer-reviewed, 10 authors. | risk of death, 6.9% higher, RR 1.07, p = 0.88, treatment 44 of 568 (7.7%), control 15 of 207 (7.2%). |
| [Almazrou], 10/1/2020, retrospective, Saudi Arabia, Middle East, peer-reviewed, 5 authors. | risk of mechanical ventilation, 65.0% lower, RR 0.35, p = 0.16, treatment 3 of 95 (3.2%), control 6 of 66 (9.1%). |
| risk of ICU admission, 21.0% lower, RR 0.79, p = 0.78, treatment 8 of 95 (8.4%), control 7 of 66 (10.6%). | |
| [Alqassieh], 12/10/2020, prospective, Jordan, Middle East, preprint, 10 authors. | hospitalization time, 18.2% lower, relative time 0.82, p = 0.11, treatment 63, control 68. |
| [An], 7/7/2020, retrospective, South Korea, Asia, preprint, 12 authors. | time to viral clearance, 3.0% lower, RR 0.97, p = 0.92, treatment 31, control 195. |
| [Annie], 10/12/2020, retrospective, database analysis, USA, North America, peer-reviewed, 5 authors. | risk of death, 4.3% lower, RR 0.96, p = 0.83, treatment 48 of 367 (13.1%), control 50 of 367 (13.6%), odds ratio converted to relative risk. |
| risk of death, 20.5% higher, RR 1.21, p = 0.46, treatment 29 of 199 (14.6%), control 24 of 199 (12.1%), odds ratio converted to relative risk. | |
| [Aparisi], 10/8/2020, prospective, Spain, Europe, preprint, 18 authors. | risk of death, 63.0% lower, RR 0.37, p = 0.008, treatment 122 of 605 (20.2%), control 27 of 49 (55.1%). |
| [Arshad], 7/1/2020, retrospective, USA, North America, peer-reviewed, 12 authors. | risk of death, 51.3% lower, RR 0.49, p = 0.009, treatment 162 of 1202 (13.5%), control 108 of 409 (26.4%). |
| [Ashinyo], 9/15/2020, retrospective, Ghana, Africa, peer-reviewed, 16 authors. | hospitalization time, 33.0% lower, relative time 0.67, p = 0.03, treatment 61, control 61. |
| [Auld], 4/26/2020, retrospective, USA, North America, peer-reviewed, 14 authors. | risk of death, 2.8% higher, RR 1.03, p = 1.00, treatment 33 of 114 (28.9%), control 29 of 103 (28.2%). |
| [Awad], 2/18/2021, retrospective, USA, North America, peer-reviewed, 4 authors | peer-reviewed, 4 authors. Submit Corrections or Updates. risk of death, 19.1% higher, RR 1.19, p = 0.60, treatment 56 of 188 (29.8%), control 37 of 148 (25.0%). |
| risk of mechanical ventilation, 460.7% higher, RR 5.61, p < 0.001, treatment 64 of 188 (34.0%), control 9 of 148 (6.1%), adjusted per study, odds ratio converted to relative risk. | |
| risk of ICU admission, 463.4% higher, RR 5.63, p < 0.001, treatment 67 of 188 (35.6%), control 9 of 148 (6.1%), adjusted per study, odds ratio converted to relative risk. | |
| [Ayerbe], 9/30/2020, retrospective, database analysis, Spain, Europe, peer-reviewed, 3 authors. | risk of death, 52.2% lower, RR 0.48, p < 0.001, treatment 237 of 1857 (12.8%), control 49 of 162 (30.2%), adjusted per study, odds ratio converted to relative risk. |
| [Barbosa], 4/12/2020, retrospective, USA, North America, preprint, 5 authors. | risk of death, 147.0% higher, RR 2.47, p = 0.58, treatment 2 of 17 (11.8%), control 1 of 21 (4.8%). |
| [Barry], 3/23/2021, retrospective, Saudi Arabia, Middle East, peer-reviewed, 14 authors. | risk of death, 98.9% lower, RR 0.01, p = 0.60, treatment 0 of 6 (0.0%), control 91 of 599 (15.2%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| [Beltran-Gonzalez], 2/23/2021, Double Blind Randomized Controlled Trial, Mexico, North America, peer-reviewed, mean age 53.8, 13 authors. | risk of death, 62.6% lower, RR 0.37, p = 0.27, treatment 2 of 33 (6.1%), control 6 of 37 (16.2%). |
| risk of respiratory deterioration or death, 25.3% lower, RR 0.75, p = 0.57, treatment 6 of 33 (18.2%), control 9 of 37 (24.3%). | |
| risk of no hospital discharge, 12.1% higher, RR 1.12, p = 1.00, treatment 3 of 33 (9.1%), control 3 of 37 (8.1%). | |
| [Berenguer], 8/3/2020, retrospective, Spain, Europe, peer-reviewed, 8 authors | risk of death, 61.9% lower, RR 0.38, p < 0.001, treatment 681 of 2618 (26.0%), control 939 of 1377 (68.2%). |
| [Bernaola], 7/21/2020, retrospective, Spain, Europe, preprint, 7 authors. | risk of death, 17.0% lower, RR 0.83, p < 0.001, treatment 236 of 1498 (15.8%), control 28 of 147 (19.0%). |
| [Bielza], 12/11/2020, retrospective, Spain, Europe, peer-reviewed, median age 87.0, 24 authors | risk of death, 21.5% lower, RR 0.78, p = 0.09, treatment 33 of 91 (36.3%), control 249 of 539 (46.2%). |
| [Boari], 11/17/2020, retrospective, Italy, Europe, peer-reviewed, 20 authors. | risk of death, 54.5% lower, RR 0.45, p < 0.001, treatment 41 of 202 (20.3%), control 25 of 56 (44.6%). |
| [Bousquet], 6/23/2020, prospective, France, Europe, peer-reviewed, 10 authors. | risk of death, 42.8% lower, RR 0.57, p = 0.15, treatment 5 of 27 (18.5%), control 23 of 81 (28.4%), adjusted per study, odds ratio converted to relative risk. |
| [Budhiraja], 11/18/2020, retrospective, India, South Asia, preprint, 12 authors | risk of death, 65.4% lower, RR 0.35, p < 0.001, treatment 69 of 834 (8.3%), control 34 of 142 (23.9%). |
| [Cangiano], 12/22/2020, retrospective, Italy, Europe, peer-reviewed, 14 authors. | risk of death, 73.4% lower, RR 0.27, p = 0.03, treatment 5 of 33 (15.2%), control 37 of 65 (56.9%). |
| [Capsoni], 12/1/2020, retrospective, Italy, Europe, preprint, 13 authors. | risk of mechanical ventilation, 40.0% lower, RR 0.60, p = 0.30, treatment 12 of 40 (30.0%), control 6 of 12 (50.0%). |
| [Catteau], 8/24/2020, retrospective, database analysis, Belgium, Europe, peer-reviewed, 11 authors | risk of death, 32.0% lower, RR 0.68, p < 0.001, treatment 804 of 4542 (17.7%), control 957 of 3533 (27.1%). |
| [Cavalcanti], 7/23/2020, Randomized Controlled Trial, Brazil, South America, peer-reviewed, baseline oxygen requirements 41.8%, 14 authors. | risk of death, 16.0% lower, RR 0.84, p = 0.77, treatment 8 of 331 (2.4%), control 5 of 173 (2.9%), HCQ+HCQ/AZ. |
| risk of hospitalization, 28.0% higher, RR 1.28, p = 0.30, treatment 331, control 173, HCQ+HCQ/AZ. | |
| Chari], 12/24/2020, retrospective, multiple countries, multiple regions, peer-reviewed, median age 69.0, 25 authors. | risk of death, 33.1% lower, RR 0.67, p = 0.17, treatment 8 of 29 (27.6%), control 195 of 473 (41.2%). |
| [Chen (B)], 7/10/2020, Randomized Controlled Trial, Taiwan, Asia, peer-reviewed, 19 authors. | risk of no virological cure, 24.0% lower, RR 0.76, p = 0.71, treatment 4 of 21 (19.0%), control 3 of 12 (25.0%), day 14. |
| median time to PCR-, 50.0% lower, relative time 0.50, p = 0.40, treatment 21, control 12. | |
| [Chen (C)], 7/10/2020, retrospective, Taiwan, Asia, peer-reviewed, 19 authors. | risk of no virological cure, 29.0% higher, RR 1.29, p = 0.70, treatment 16 of 28 (57.1%), control 4 of 9 (44.4%), day 14. |
| [Chen (D)], 3/31/2020, Randomized Controlled Trial, China, Asia, preprint, 9 authors. | risk of no improvement in pneumonia at day 6, 57.0% lower, RR 0.43, p = 0.04, treatment 6 of 31 (19.4%), control 14 of 31 (45.2%). |
| [Chen (E)], 3/6/2020, Randomized Controlled Trial, China, Asia, peer-reviewed, 14 authors. | risk of radiological progression, 29.0% lower, RR 0.71, p = 0.57, treatment 5 of 15 (33.3%), control 7 of 15 (46.7%). |
| risk of viral+ at day 7, 100% higher, RR 2.00, p = 1.00, treatment 2 of 15 (13.3%), control 1 of 15 (6.7%). | |
| [Choi], 10/27/2020, retrospective, database analysis, South Korea, Asia, peer-reviewed, 8 authors. | median time to PCR-, 22.0% higher, relative time 1.22, p < 0.001, treatment 701, control 701. |
| [Coll], 10/23/2020, retrospective, Spain, Europe, peer-reviewed, median age 61.0, 29 authors | risk of death, 45.6% lower, RR 0.54, p < 0.001, treatment 55 of 307 (17.9%), control 108 of 328 (32.9%). |
| [Cravedi], 7/10/2020, retrospective, USA, multiple countries, North America, multiple regions, peer-reviewed, mean age 60.0, 25 authors. | risk of death, 53.0% higher, RR 1.53, p = 0.17, treatment 36 of 101 (35.6%), control 10 of 43 (23.3%). |
| [D’Arminio Monforte], 7/29/2020, retrospective, Italy, Europe, preprint, 5 authors | risk of death, 34.0% lower, RR 0.66, p = 0.12, treatment 53 of 197 (26.9%), control 47 of 92 (51.1%), adjusted per study. |
| [Davido], 8/2/2020, retrospective, France, Europe, peer-reviewed, 14 authors. | risk of combined intubation/hospitalization, 55.0% lower, RR 0.45, p = 0.04, treatment 12 of 80 (15.0%), control 13 of 40 (32.5%). |
| [Di Castelnuovo], 8/25/2020, retrospective, Italy, Europe, peer-reviewed, 110 authors | risk of death, 30.0% lower, RR 0.70, p < 0.001, treatment 386 of 2634 (14.7%), control 90 of 817 (11.0%), adjusted per study. |
| [Dubee], 10/21/2020, Randomized Controlled Trial, France, Europe, peer-reviewed, median age 77.0, 18 authors. | risk of death at day 28, 46.0% lower, RR 0.54, p = 0.21, treatment 6 of 124 (4.8%), control 11 of 123 (8.9%). |
| risk of combined intubation/death at day 28, 26.0% lower, RR 0.74, p = 0.48, treatment 9 of 124 (7.3%), control 12 of 123 (9.8%). | |
| [Dubernet], 8/20/2020, retrospective, France, Africa, peer-reviewed, median age 66.0, 20 authors | risk of ICU admission, 87.6% lower, RR 0.12, p = 0.008, treatment 1 of 17 (5.9%), control 9 of 19 (47.4%). |
| [Falcone], 11/19/2020, prospective, Italy, Europe, peer-reviewed, 19 authors. | reviewed, 19 authors. Submit Corrections or Updates. risk of death, 65.0% lower, RR 0.35, p = 0.20, treatment 40 of 238 (16.8%), control 30 of 77 (39.0%), adjusted per study, PSM. |
| risk of death, 25.0% lower, RR 0.75, p = 0.36, treatment 40 of 238 (16.8%), control 30 of 77 (39.0%), adjusted per study, multivariate Cox regression. | |
| risk of death, 57.0% lower, RR 0.43, p < 0.001, treatment 40 of 238 (16.8%), control 30 of 77 (39.0%), adjusted per study, univariate Cox regression. | |
| [Faíco-Filho], 6/21/2020, prospective, Brazil, South America, peer-reviewed, median age 58.0, 6 authors. | Δt7-12 ΔCt improvement, 80.8% lower, relative rate 0.19, p = 0.40, treatment 34, control 32. |
| Δt<7 ΔCt improvement, 24.0% lower, relative rate 0.76, p = 0.36, treatment 34, control 32. | |
| Δt>12 ΔCt improvement, 15.0% higher, relative rate 1.15, p = 0.52, treatment 34, control 32. | |
| [Fontana], 6/22/2020, retrospective, Italy, Europe, peer-reviewed, 8 authors | risk of death, 50.0% lower, RR 0.50, p = 0.53, treatment 4 of 12 (33.3%), control 2 of 3 (66.7%). |
| [Fried], 8/28/2020, retrospective, database analysis, USA, North America, peer-reviewed, 11 authors. | risk of death, 27.0% higher, RR 1.27, p < 0.001, treatment 1048 of 4232 (24.8%), control 1466 of 7489 (19.6%). |
| [Frontera], 10/26/2020, retrospective, USA, North America, preprint, median age 64.0, 14 authors. | risk of death, 37.0% lower, RR 0.63, p = 0.01, treatment 121 of 1006 (12.0%), control 424 of 2467 (17.2%), adjusted per study, PSM. |
| risk of death, 24.0% lower, RR 0.76, p = 0.02, treatment 121 of 1006 (12.0%), control 424 of 2467 (17.2%), adjusted per study, regression. | |
| [Geleris], 5/7/2020, retrospective, USA, North America, peer-reviewed, 12 authors. | risk of combined intubation/death, 4.0% higher, RR 1.04, p = 0.76, treatment 262 of 811 (32.3%), control 84 of 565 (14.9%), adjusted per study. |
| [Goldman], 5/27/2020, retrospective, multiple countries, multiple regions, peer-reviewed, 26 authors. | risk of death, 22.3% lower, RR 0.78, p = 0.46, treatment 10 of 109 (9.2%), control 34 of 288 (11.8%). |
| [Gonzalez], 8/21/2020, retrospective, database analysis, Spain, Europe, preprint, 25 authors. | risk of death, 26.6% lower, RR 0.73, p = 0.06, treatment 1246 of 8476 (14.7%), control 341 of 1168 (29.2%), adjusted per study, odds ratio converted to relative risk. |
| [Guglielmetti], 12/9/2020, retrospective, Italy, Europe, peer-reviewed, 16 authors. | risk of death, 35.0% lower, RR 0.65, p = 0.22, treatment 181, control 37, adjusted per study, multivariable Cox. |
| [Guisado-Vasco (B)], 10/15/2020, retrospective, Spain, Europe, peer-reviewed, median age 69.0, 25 authors. | risk of death, 20.3% lower, RR 0.80, p = 0.36, treatment 127 of 558 (22.8%), control 14 of 49 (28.6%), adjusted per study, odds ratio converted to relative risk. |
| [Gupta], 7/15/2020, retrospective, USA, North America, peer-reviewed, baseline oxygen requirements 87.1%, 34 authors | risk of death, 6.0% higher, RR 1.06, p = 0.41, treatment 631 of 1761 (35.8%), control 153 of 454 (33.7%). |
| [Güner], 12/29/2020, retrospective, Turkey, Middle East, peer-reviewed, 23 authors. | risk of ICU admission, 77.3% lower, RR 0.23, p = 0.16, treatment 604, control 100, IPTW multivariate analysis. |
| [Heberto], 9/12/2020, prospective, Mexico, North America, peer-reviewed, 8 authors | risk of death, 53.6% lower, RR 0.46, p = 0.04, treatment 139, control 115, odds ratio converted to relative risk. |
| risk of mechanical ventilation, 65.6% lower, RR 0.34, p = 0.008, odds ratio converted to relative risk. | |
| [Hernandez-Cardenas], 2/5/2021, Randomized Controlled Trial, Mexico, North America, preprint, 6 authors | risk of death, 12.0% lower, RR 0.88, p = 0.66, treatment 106, control 108. |
| risk of death, 57.0% lower, RR 0.43, p = 0.29, subgroup not intubated at baseline. | |
| [Hraiech], 5/24/2020, retrospective, France, Europe, peer-reviewed, 8 authors. | risk of death, 64.7% lower, RR 0.35, p = 0.21, treatment 2 of 17 (11.8%), control 5 of 15 (33.3%), day 38 +- 7. |
| risk of death, 376.5% higher, RR 4.76, p = 0.49, treatment 2 of 17 (11.8%), control 0 of 15 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 6 from ARDS. | |
| risk of no virological cure, 2.9% higher, RR 1.03, p = 1.00, treatment 14 of 17 (82.4%), control 8 of 10 (80.0%), day 6 from treatment. | |
| [Huang (C)], 5/28/2020, prospective, China, Asia, peer-reviewed, 36 authors. | time to viral-, 67.0% lower, relative time 0.33, p < 0.001, treatment 197, control 176. |
| time to viral-, 59.1% lower, relative time 0.41, p < 0.001, treatment 32, control 37, early treatment. | |
| [Ip (B)], 5/25/2020, retrospective, database analysis, USA, North America, peer-reviewed, 32 authors. | risk of death, 1.0% lower, RR 0.99, p = 0.93, treatment 432 of 1914 (22.6%), control 115 of 598 (19.2%), adjusted per study. |
| [Johnston], 12/9/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 30 authors, dosage 400mg bid day 1, 200mg bid days 2-10 | risk of hospitalization, 29.9% lower, RR 0.70, p = 0.73, treatment 5 of 148 (3.4%), control 4 of 83 (4.8%), HCQ + folic acid and HCQ + AZ vs. vitamin C + folic acid. |
| risk of no recovery, 2.0% lower, RR 0.98, p = 0.95, treatment 30 of 60 (50.0%), control 34 of 72 (47.2%), adjusted per study, HCQ + folic acid vs. vitamin C + folic acid. | |
| risk of no recovery, 9.9% higher, RR 1.10, p = 0.70, treatment 34 of 65 (52.3%), control 34 of 72 (47.2%), adjusted per study, HCQ + AZ vs. vitamin C + folic acid. | |
| time to viral-, 28.6% lower, relative time 0.71, treatment 49, control 52, median time, HCQ + folic acid vs. vitamin C + folic acid. | |
| time to viral-, 14.3% lower, relative time 0.86, treatment 51, control 52, median time, HCQ + AZ vs. vitamin C + folic acid. | |
| risk of no virological cure, 38.3% lower, RR 0.62, p = 0.05, treatment 6 of 49 (12.2%), control 12 of 52 (23.1%), adjusted per study, HCQ + folic acid vs. vitamin C + folic acid. | |
| risk of no virological cure, 20.0% lower, RR 0.80, p = 0.49, treatment 11 of 51 (21.6%), control 12 of 52 (23.1%), adjusted per study, HCQ + AZ vs. vitamin C + folic acid. | |
| [Kalligeros], 8/5/2020, retrospective, USA, North America, peer-reviewed, 13 authors | risk of death, 67.0% higher, RR 1.67, p = 0.57, treatment 36, control 72. |
| [Kamran], 8/4/2020, prospective, Pakistan, South Asia, preprint, 10 authors. | risk of disease progression, 5.0% lower, RR 0.95, p = 1.00, treatment 11 of 349 (3.2%), control 5 of 151 (3.3%). |
| risk of disease progression, 54.8% lower, RR 0.45, p = 0.30, treatment 4 of 31 (12.9%), control 2 of 7 (28.6%), with comorbidities. | |
| risk of viral+ at day 14, 10.0% higher, RR 1.10, p = 0.52, treatment 349, control 151. | |
| [Kelly], 7/22/2020, retrospective, Ireland, Europe, peer-reviewed, 14 authors. | risk of death, 143.0% higher, RR 2.43, p = 0.03, treatment 23 of 82 (28.0%), control 6 of 52 (11.5%). |
| [Kim], 5/18/2020, retrospective, South Korea, Asia, preprint, 11 authors. | hospitalization time, 51.0% lower, relative time 0.49, p = 0.01, treatment 22, control 40. |
| time to viral-, 56.0% lower, relative time 0.44, p = 0.005, treatment 22, control 40. | |
| Komissarov], 6/30/2020, retrospective, Russia, Asia, Europe, preprint, 8 authors. | risk of viral load, 25.0% higher, RR 1.25, p = 0.45, treatment 26, control 10. |
| [Kuderer], 5/28/2020, retrospective, USA, multiple countries, North America, multiple regions, peer-reviewed, 73 authors. | risk of death, 134.2% higher, RR 2.34, p < 0.001, treatment 45 of 181 (24.9%), control 121 of 928 (13.0%), odds ratio converted to relative risk, HCQ+AZ. |
| [Lamback], 2/19/2021, retrospective, Brazil, South America, peer-reviewed, 10 authors. | risk of death, 8.9% lower, RR 0.91, p = 0.83, treatment 11 of 101 (10.9%), control 11 of 92 (12.0%). |
| risk of ICU admission, 19.9% higher, RR 1.20, p = 0.61, treatment 25 of 101 (24.8%), control 19 of 92 (20.7%). | |
| hospitalization time, 12.5% lower, relative time 0.88, treatment 101, control 92. | |
| [Lambermont], 11/28/2020, retrospective, Belgium, Europe, peer-reviewed, 15 authors | risk of death, 32.3% lower, RR 0.68, p = 0.46, treatment 97 of 225 (43.1%), control 14 of 22 (63.6%), adjusted per study. |
| [Lammers], 9/29/2020, prospective, Netherlands, Europe, peer-reviewed, 18 authors | risk of combined death/ICU, 32.0% lower, RR 0.68, p = 0.02, treatment 30 of 189 (15.9%), control 101 of 498 (20.3%), adjusted per study. |
| [Lano], 10/21/2020, retrospective, France, Europe, peer-reviewed, median age 73.5, 30 authors. | risk of death, 33.1% lower, RR 0.67, p = 0.28, treatment 56, control 66, adjusted per study, odds ratio converted to relative risk. |
| risk of combined death/ICU, 38.9% lower, RR 0.61, p = 0.23, treatment 17 of 56 (30.4%), control 28 of 66 (42.4%), adjusted per study, odds ratio converted to relative risk. | |
| risk of combined death/ICU, 68.7% lower, RR 0.31, p = 0.11, treatment 4 of 36 (11.1%), control 11 of 31 (35.5%), not requiring O2 on diagnosis (relatively early treatment). | |
| [Lauriola], 9/14/2020, retrospective, Italy, Europe, peer-reviewed, mean age 71.8, 10 authors. | risk of death, 73.5% lower, RR 0.27, p < 0.001, treatment 102 of 297 (34.3%), control 35 of 63 (55.6%), adjusted per study. |
| [Lecronier], 7/11/2020, retrospective, France, Europe, peer-reviewed, baseline oxygen requirements 100.0%, 25 authors, HCQ vs. control | risk of death, 42.0% lower, RR 0.58, p = 0.24, treatment 9 of 38 (23.7%), control 9 of 22 (40.9%). |
| risk of treatment escalation, 6.0% lower, RR 0.94, p = 0.73, treatment 15 of 38 (39.5%), control 9 of 22 (40.9%). | |
| risk of viral+ at day 7, 15.0% lower, RR 0.85, p = 0.61, treatment 19 of 26 (73.1%), control 12 of 14 (85.7%). | |
| [Li], 1/18/2021, retrospective, China, Asia, peer-reviewed, 21 authors. | risk of no hospital discharge, 50.0% lower, RR 0.50, p = 0.08, treatment 14, control 14, RCT patients vs. matched sample of non-treated patients. |
| [Li (B)], 1/12/2021, retrospective, database analysis, China, Asia, preprint, 5 authors. | time to viral-, 40.0% higher, relative time 1.40, p = 0.06, treatment 18, control 19. |
| [Lora-Tamayo], 2/11/2021, retrospective, Spain, Europe, peer-reviewed, 10 authors. | risk of death, 50.5% lower, RR 0.50, p < 0.001, treatment 7192, control 1361, odds ratio converted to relative risk, univariate. |
| [Lotfy], 1/1/2021, retrospective, Saudi Arabia, Middle East, peer-reviewed, mean age 55.0, 3 authors. | risk of death, 24.8% higher, RR 1.25, p = 0.76, treatment 6 of 99 (6.1%), control 5 of 103 (4.9%). |
| risk of mechanical ventilation, 41.2% higher, RR 1.41, p = 0.34, treatment 19 of 99 (19.2%), control 14 of 103 (13.6%). | |
| risk of ICU admission, 16.5% higher, RR 1.17, p = 0.53, treatment 28 of 99 (28.3%), control 25 of 103 (24.3%). | |
| [Luo], 6/17/2020, retrospective, USA, North America, peer-reviewed, 31 authors. | risk of death, 2.2% higher, RR 1.02, p = 0.99, treatment 11 of 35 (31.4%), control 4 of 13 (30.8%), odds ratio converted to relative risk. |
| [Lyngbakken], 7/17/2020, Randomized Controlled Trial, Norway, Europe, peer-reviewed, median age 62.0, 11 authors. | risk of death, 3.7% lower, RR 0.96, p = 1.00, treatment 1 of 27 (3.7%), control 1 of 26 (3.8%). |
| improvement in viral load reduction rate, 71.0% lower, relative rate 0.29, p = 0.51, treatment 27, control 26. | |
| [López], 11/2/2020, retrospective, Spain, Europe, peer-reviewed, 7 authors. | risk of disease progression, 64.3% lower, RR 0.36, p = 0.02, treatment 5 of 36 (13.9%), control 14 of 36 (38.9%). |
| [Magagnoli], 4/21/2020, retrospective, database analysis, USA, North America, peer-reviewed, 7 authors. | risk of death, 11.0% lower, RR 0.89, p = 0.74, treatment 39 of 148 (26.4%), control 18 of 163 (11.0%), adjusted per study, HCQ+AZ w/dispositions. |
| risk of death, 1.0% lower, RR 0.99, p = 0.98, treatment 30 of 114 (26.3%), control 18 of 163 (11.0%), adjusted per study, HCQ w/dispositions. | |
| risk of death, 31.0% higher, RR 1.31, p = 0.28, treatment 49 of 214 (22.9%), control 37 of 395 (9.4%), adjusted per study, HCQ+AZ. | |
| risk of death, 83.0% higher, RR 1.83, p = 0.009, treatment 38 of 198 (19.2%), control 37 of 395 (9.4%), adjusted per study, HCQ. | |
| [Mahévas], 5/14/2020, retrospective, France, Europe, peer-reviewed, 34 authors. | risk of death, 20.0% higher, RR 1.20, p = 0.75, treatment 9 of 84 (10.7%), control 8 of 89 (9.0%), adjusted per study. |
| [Maldonado], 11/5/2020, retrospective, Spain, Europe, peer-reviewed, 10 authors. | risk of death, 90.9% lower, RR 0.09, p = 0.17, treatment 1 of 11 (9.1%), control 1 of 1 (100.0%). |
| [Mallat], 5/2/2020, retrospective, Abu Dhabi, Middle East, peer-reviewed, 8 authors. | time to viral-, 203.0% higher, relative time 3.03, p = 0.02, treatment 23, control 11. |
| [Martin-Vicente], 3/8/2021, retrospective, Spain, Europe, preprint, 38 authors. | risk of death, 59.3% lower, RR 0.41, p = 0.41, treatment 37 of 91 (40.7%), control 1 of 1 (100.0%). |
| [Martinez-Lopez], 6/30/2020, retrospective, Spain, Europe, peer-reviewed, median age 71.0, 25 authors. | risk of death, 33.0% lower, RR 0.67, p = 0.20, treatment 47 of 148 (31.8%), control 9 of 19 (47.4%). |
| [Matangila], 12/18/2020, retrospective, DR Congo, Africa, peer-reviewed, median age 54.0, 12 authors. | risk of death, 54.9% lower, RR 0.45, p = 0.21, treatment 25 of 147 (17.0%), control 8 of 13 (61.5%), adjusted per study, odds ratio converted to relative risk. |
| [McGrail], 7/19/2020, retrospective, USA, North America, preprint, 2 authors. | risk of death, 70.0% higher, RR 1.70, p = 0.69, treatment 4 of 33 (12.1%), control 3 of 42 (7.1%). |
| [Membrillo de Novales], 5/5/2020, retrospective, Spain, Europe, preprint, 19 authors. | risk of death, 55.1% lower, RR 0.45, p = 0.002, treatment 27 of 123 (22.0%), control 21 of 43 (48.8%). |
| [Mikami], 6/30/2020, retrospective, USA, North America, peer-reviewed, 7 authors. | risk of death, 47.0% lower, RR 0.53, p < 0.001, treatment 575 of 2077 (27.7%), control 231 of 743 (31.1%), adjusted per study. |
| [Modrák], 12/4/2020, retrospective, Czech Republic, Europe, preprint, 26 authors. | risk of death, 59.0% lower, RR 0.41, p = 0.04, treatment 108, control 105, Cox (single). |
| [Nachega], 10/2/2020, retrospective, database analysis, Democratic Republic of Congo, Africa, peer-reviewed, median age 46.0, 25 authors. | risk of death, 27.6% lower, RR 0.72, p = 0.17, treatment 69 of 630 (11.0%), control 28 of 96 (29.2%), adjusted per study, odds ratio converted to relative risk. |
| risk of no improvement, 25.8% lower, RR 0.74, p = 0.13, adjusted per study, odds ratio converted to relative risk. | |
| [Naseem], 12/14/2020, retrospective, Pakistan, South Asia, preprint, 5 authors. | risk of death, 33.3% lower, RR 0.67, p = 0.34, treatment 77, control 1137, multivariate Cox. |
| [Núñez-Gil], 11/9/2020, retrospective, database analysis, multiple countries, Europe, peer-reviewed, median age 68.0, 49 authors. | risk of death, 7.9% lower, RR 0.92, p = 0.005, treatment 200 of 686 (29.2%), control 100 of 268 (37.3%), adjusted per study, odds ratio converted to relative risk. |
| [Orioli], 12/14/2020, retrospective, Belgium, Europe, peer-reviewed, 9 authors. | risk of death, 12.7% lower, RR 0.87, p = 1.00, treatment 8 of 55 (14.5%), control 3 of 18 (16.7%). |
| [Ouedraogo], 2/5/2021, retrospective, Burkina Faso, Africa, peer-reviewed, 14 authors. | risk of death, 33.0% lower, RR 0.67, p = 0.38, treatment 397, control 59, multivariate. |
| risk of ARDS, 68.0% lower, RR 0.32, p = 0.001, treatment 397, control 59, multivariate. | |
| [Ozturk], 12/4/2020, retrospective, Turkey, Middle East, peer-reviewed, 70 authors. | risk of death, 43.9% lower, RR 0.56, p = 0.14, treatment 165 of 1127 (14.6%), control 6 of 23 (26.1%), CQ/HCQ. |
| [Paccoud], 6/18/2020, retrospective, France, Europe, peer-reviewed, 20 authors. | risk of death, 11.0% lower, RR 0.89, p = 0.88, treatment 21 of 38 (55.3%), control 26 of 46 (56.5%), adjusted per study. |
| [Pasquini], 8/23/2020, retrospective, Italy, Europe, peer-reviewed, 9 authors | risk of death, 16.4% lower, RR 0.84, p = 0.34, treatment 23 of 33 (69.7%), control 15 of 18 (83.3%). |
| [Peng], 12/4/2020, retrospective, China, Asia, peer-reviewed, 21 authors. | risk of disease progression, 10.8% lower, RR 0.89, p = 0.63, treatment 29 of 453 (6.4%), control 256 of 3567 (7.2%), CQ/HCQ risk of AKI. |
| [Peters], 8/15/2020, retrospective, Netherlands, Europe, peer-reviewed, 21 authors | risk of death, 9.0% higher, RR 1.09, p = 0.57, treatment 419 of 1596 (26.3%), control 53 of 353 (15.0%), adjusted per study. |
| [Pinato], 8/18/2020, retrospective, multiple countries, Europe, peer-reviewed, 64 authors. | risk of death, 59.0% lower, RR 0.41, p < 0.001, treatment 30 of 182 (16.5%), control 181 of 446 (40.6%). |
| [Psevdos], 12/31/2020, retrospective, USA, North America, peer-reviewed, 3 authors. | America, peer-reviewed, 3 authors. Submit Corrections or Updates. risk of death, 63.5% higher, RR 1.63, p = 0.52, treatment 17 of 52 (32.7%), control 3 of 15 (20.0%). |
| [Purwati], 2/9/2021, Double Blind Randomized Controlled Trial, Indonesia, Asia, peer-reviewed, 12 authors. | risk of no virological cure, 66.3% lower, RR 0.34, p < 0.001, treatment 38 of 121 (31.4%), control 111 of 119 (93.3%). |
| [Qin], 11/23/2020, retrospective, China, Asia, peer-reviewed, 17 authors. | risk of death, 34.3% lower, RR 0.66, p = 0.61, treatment 3 of 43 (7.0%), control 75 of 706 (10.6%). |
| [RECOVERY], 6/5/2020, Randomized Controlled Trial, United Kingdom, Europe, preprint, 29 authors | risk of death, 9.0% higher, RR 1.09, p = 0.15, treatment 421 of 1561 (27.0%), control 790 of 3155 (25.0%). |
| [Rivera], 7/22/2020, retrospective, USA, North America, peer-reviewed, 45 authors. | risk of death, 2.4% higher, RR 1.02, p = 0.90, treatment 44 of 179 (24.6%), control 59 of 327 (18.0%), adjusted per study, odds ratio converted to relative risk. |
| [Rivera-Izquierdo], 7/9/2020, retrospective, Spain, Europe, peer-reviewed, 21 authors | risk of death, 19.0% lower, RR 0.81, p = 0.75, treatment 215, control 23. |
| [Rodriguez], 11/9/2020, prospective, Spain, Europe, peer-reviewed, 13 authors. | risk of death, 59.0% lower, RR 0.41, p = 0.23, treatment 8 of 39 (20.5%), control 2 of 4 (50.0%). |
| [Rodriguez-Gonzalez], 11/28/2020, retrospective, Spain, Europe, peer-reviewed, 20 authors | risk of death, 22.8% lower, RR 0.77, p = 0.26, treatment 251 of 1148 (21.9%), control 17 of 60 (28.3%). |
| [Rodriguez-Nava], 11/5/2020, retrospective, USA, North America, peer-reviewed, median age 68.0, 8 authors. | risk of death, 6.3% higher, RR 1.06, p = 0.77, treatment 22 of 65 (33.8%), control 79 of 248 (31.9%), unadjusted. |
| [Roig], 1/31/2021, retrospective, Spain, Europe, peer-reviewed, 6 authors. | risk of death, 15.6% lower, RR 0.84, p = 0.76, treatment 33 of 67 (49.3%), control 7 of 12 (58.3%). |
| [Roomi], 8/13/2020, retrospective, USA, North America, peer-reviewed, 11 authors. | risk of death, 37.7% higher, RR 1.38, p = 0.54, treatment 13 of 144 (9.0%), control 6 of 32 (18.8%), adjusted per study, odds ratio converted to relative risk. |
| [Rosenberg], 5/11/2020, retrospective, USA, North America, peer-reviewed, 14 authors. | risk of death, 35.0% higher, RR 1.35, p = 0.31, treatment 189 of 735 (25.7%), control 28 of 221 (12.7%), adjusted per study. |
| [Salazar], 11/4/2020, retrospective, USA, North America, peer-reviewed, 19 authors. | risk of death, 37.0% higher, RR 1.37, p = 0.28, treatment 12 of 92 (13.0%), control 80 of 811 (9.9%). |
| [Saleemi], 8/11/2020, retrospective, Saudi Arabia, Middle East, preprint, 5 authors | Middle East, preprint, 5 authors. Submit Corrections or Updates. median time to PCR-, 21.0% higher, relative time 1.21, p < 0.05, treatment 65, control 20. |
| [Salvador], 3/4/2021, prospective, Portugal, Europe, peer-reviewed, 10 authors. | risk of death, 32.9% lower, RR 0.67, p = 0.007, treatment 28 of 121 (23.1%), control 58 of 124 (46.8%), odds ratio converted to relative risk, multivariate. |
| risk of mechanical ventilation, 447.8% higher, RR 5.48, p = 0.003, treatment 32 of 121 (26.4%), control 12 of 124 (9.7%), odds ratio converted to relative risk, multivariate. | |
| risk of combined intubation/death, 16.7% lower, RR 0.83, p = 0.02, treatment 51 of 121 (42.1%), control 63 of 124 (50.8%), odds ratio converted to relative risk, univariate. | |
| [Sands], 1/1/2021, retrospective, database analysis, USA, North America, peer-reviewed, 10 authors. | risk of death, 69.9% higher, RR 1.70, p = 0.01, treatment 101 of 973 (10.4%), control 56 of 696 (8.0%), odds ratio converted to relative risk. |
| [Sarfaraz], 1/2/2021, retrospective, Pakistan, South Asia, preprint, 7 authors. | risk of death, 45.0% higher, RR 1.45, p = 0.07, treatment 40 of 94 (42.6%), control 27 of 92 (29.3%). |
| [Sbidian], 6/19/2020, retrospective, database analysis, France, Europe, preprint, 21 authors, whole population HCQ AIPTW adjusted. | risk of death, 5.0% higher, RR 1.05, p = 0.74, treatment 111 of 623 (17.8%), control 830 of 3792 (21.9%), adjusted per study. |
| risk of no hospital discharge, 20.0% lower, RR 0.80, p = 0.002, adjusted per study. | |
| [Self], 11/9/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 33 authors | risk of death, 6.2% higher, RR 1.06, p = 0.84, treatment 25 of 241 (10.4%), control 25 of 236 (10.6%), adjusted per study, odds ratio converted to relative risk. |
| [Serrano], 9/22/2020, retrospective, Spain, Europe, peer-reviewed, 8 authors. | risk of death, 43.0% lower, RR 0.57, p = 0.14, treatment 6 of 14 (42.9%), control 6 of 8 (75.0%). |
| Shabrawishi], 5/11/2020, retrospective, Saudi Arabia, Middle East, preprint, mean age 43.9, 5 authors. | risk of no virological cure at day 5, 14.7% lower, RR 0.85, p = 0.66, treatment 12 of 45 (26.7%), control 15 of 48 (31.2%). |
| [Sheshah], 11/13/2020, retrospective, Saudi Arabia, Middle East, peer-reviewed, 8 authors. | risk of death, 80.0% lower, RR 0.20, p < 0.001, treatment 267, control 33, odds ratio converted to relative risk. |
| [Shoaibi], 9/24/2020, retrospective, database analysis, USA, North America, preprint, 5 authors. | risk of death, 15.4% lower, RR 0.85, p < 0.001, treatment 686 of 5047 (13.6%), control 3923 of 24404 (16.1%). |
| [Signes-Costa], 12/16/2020, retrospective, Spain, Canada, China, Cuba, Ecuador, Germany, Italy, Europe, Asia, Caribbean, North America, South America, peer-reviewed, 28 authors. | risk of death, 47.0% lower, RR 0.53, p < 0.001, treatment 4854, control 993, adjusted per study. |
| [Singh], 5/19/2020, retrospective, database analysis, USA, North America, preprint, 4 author | risk of death, 5.0% lower, RR 0.95, p = 0.72, treatment 104 of 910 (11.4%), control 109 of 910 (12.0%) |
| risk of mechanical ventilation, 19.0% lower, RR 0.81, p = 0.26, treatment 46 of 910 (5.1%), control 57 of 910 (6.3%). | |
| [Solh], 10/20/2020, retrospective, database analysis, USA, North America, preprint, 5 authors. | risk of death, 18.0% higher, RR 1.18, p = 0.17, treatment 131 of 265 (49.4%), control 134 of 378 (35.4%), adjusted per study. |
| [SOLIDARITY], 10/15/2020, Randomized Controlled Trial, multiple countries, multiple regions, peer-reviewed, baseline oxygen requirements 64.0%, 15 authors. | risk of death, 19.0% higher, RR 1.19, p = 0.23, treatment 104 of 947 (11.0%), control 84 of 906 (9.3%). |
| [Sosa-García], 6/29/2020, retrospective, Mexico, North America, peer-reviewed, baseline oxygen requirements 100.0%, 6 authors. | risk of death, 10.5% higher, RR 1.11, p = 1.00, treatment 7 of 38 (18.4%), control 3 of 18 (16.7%). |
| [Soto-Becerra], 10/8/2020, retrospective, database analysis, Peru, South America, preprint, median age 59.4, 4 authors. | risk of death, 18.1% lower, RR 0.82, p < 0.001, treatment 346 of 692 (50.0%), control 1606 of 2630 (61.1%), day 54 (last day available) weighted KM. |
| risk of death, 84.0% higher, RR 1.84, p = 0.02, treatment 165 of 692 (23.8%), control 401 of 2630 (15.2%), adjusted per study, day 30. | |
| [Stewart], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors. | risk of death, 18.0% higher, RR 1.18, p = 0.27, treatment 90 of 429 (21.0%), control 141 of 737 (19.1%), adjusted per study, VA, HCQ+AZ. |
| risk of death, 1.0% lower, RR 0.99, p = 0.95, treatment 66 of 578 (11.4%), control 188 of 1243 (15.1%), adjusted per study, TriNetX, HCQ+AZ. | |
| risk of death, 129.9% higher, RR 2.30, p < 0.001, treatment 32 of 108 (29.6%), control 33 of 256 (12.9%), Synapse, HCQ+AZ. | |
| risk of death, 9.0% higher, RR 1.09, p = 0.65, treatment 212 of 1157 (18.3%), control 203 of 1101 (18.4%), adjusted per study, Health Catalyst, HCQ+AZ. | |
| risk of death, 90.0% higher, RR 1.90, p = 0.09, treatment 46 of 208 (22.1%), control 47 of 1334 (3.5%), adjusted per study, Dascena, HCQ+AZ. | |
| risk of death, 16.0% higher, RR 1.16, p = 0.26, treatment 428 of 1711 (25.0%), control 123 of 688 (17.9%), adjusted per study, COTA/HMH, HCQ+AZ. | |
| risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. | |
| [Stewart (B)], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors. | risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. |
| [Stewart (C)], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors | risk of death, 16.0% higher, RR 1.16, p = 0.26, treatment 428 of 1711 (25.0%), control 123 of 688 (17.9%), adjusted per study, COTA/HMH, HCQ+AZ. |
| risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. | |
| [Stewart (D)], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors. | risk of death, 90.0% higher, RR 1.90, p = 0.09, treatment 46 of 208 (22.1%), control 47 of 1334 (3.5%), adjusted per study, Dascena, HCQ+AZ. |
| risk of death, 16.0% higher, RR 1.16, p = 0.26, treatment 428 of 1711 (25.0%), control 123 of 688 (17.9%), adjusted per study, COTA/HMH, HCQ+AZ. | |
| risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. | |
| [Stewart (E)], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors. | risk of death, 9.0% higher, RR 1.09, p = 0.65, treatment 212 of 1157 (18.3%), control 203 of 1101 (18.4%), adjusted per study, Health Catalyst, HCQ+AZ. |
| risk of death, 90.0% higher, RR 1.90, p = 0.09, treatment 46 of 208 (22.1%), control 47 of 1334 (3.5%), adjusted per study, Dascena, HCQ+AZ. | |
| risk of death, 16.0% higher, RR 1.16, p = 0.26, treatment 428 of 1711 (25.0%), control 123 of 688 (17.9%), adjusted per study, COTA/HMH, HCQ+AZ. | |
| risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. | |
| [Stewart (F)], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors | risk of death, 129.9% higher, RR 2.30, p < 0.001, treatment 32 of 108 (29.6%), control 33 of 256 (12.9%), Synapse, HCQ+AZ. |
| risk of death, 9.0% higher, RR 1.09, p = 0.65, treatment 212 of 1157 (18.3%), control 203 of 1101 (18.4%), adjusted per study, Health Catalyst, HCQ+AZ. | |
| risk of death, 90.0% higher, RR 1.90, p = 0.09, treatment 46 of 208 (22.1%), control 47 of 1334 (3.5%), adjusted per study, Dascena, HCQ+AZ. | |
| risk of death, 16.0% higher, RR 1.16, p = 0.26, treatment 428 of 1711 (25.0%), control 123 of 688 (17.9%), adjusted per study, COTA/HMH, HCQ+AZ. | |
| risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. | |
| [Stewart (G)], 3/17/2021, retrospective, USA, North America, peer-reviewed, 37 authors. | risk of death, 1.0% lower, RR 0.99, p = 0.95, treatment 66 of 578 (11.4%), control 188 of 1243 (15.1%), adjusted per study, TriNetX, HCQ+AZ. |
| risk of death, 129.9% higher, RR 2.30, p < 0.001, treatment 32 of 108 (29.6%), control 33 of 256 (12.9%), Synapse, HCQ+AZ. | |
| risk of death, 9.0% higher, RR 1.09, p = 0.65, treatment 212 of 1157 (18.3%), control 203 of 1101 (18.4%), adjusted per study, Health Catalyst, HCQ+AZ. | |
| risk of death, 90.0% higher, RR 1.90, p = 0.09, treatment 46 of 208 (22.1%), control 47 of 1334 (3.5%), adjusted per study, Dascena, HCQ+AZ. | |
| risk of death, 16.0% higher, RR 1.16, p = 0.26, treatment 428 of 1711 (25.0%), control 123 of 688 (17.9%), adjusted per study, COTA/HMH, HCQ+AZ. | |
| risk of mechanical ventilation, 29.0% higher, RR 1.29, p = 0.09, treatment 48 of 305 (15.7%), control 95 of 1302 (7.3%), adjusted per study, Aetion, HCQ. | |
| [Synolaki], 9/5/2020, retrospective, Greece, Europe, preprint, 20 authors. | risk of death, 23.6% lower, RR 0.76, p = 0.27, treatment 21 of 98 (21.4%), control 60 of 214 (28.0%). |
| [Sánchez-Álvarez], 4/27/2020, retrospective, database analysis, Spain, Europe, peer-reviewed, mean age 67.0, 10 authors | risk of death, 45.9% lower, RR 0.54, p = 0.005, treatment 322, control 53, odds ratio converted to relative risk. |
| [Taccone], 12/23/2020, retrospective, Belgium, Europe, peer-reviewed, 10 authors | risk of death, 24.7% lower, RR 0.75, p < 0.001, treatment 449 of 1308 (34.3%), control 183 of 439 (41.7%), odds ratio converted to relative risk. |
| [Tan], 12/14/2020, retrospective, China, Asia, peer-reviewed, 7 authors. | hospitalization time, 35.2% lower, relative time 0.65, p = 0.04, treatment 8, control 277. |
| [Tang], 4/14/2020, Randomized Controlled Trial, China, Asia, peer-reviewed, 24 authors. | risk of no virological cure at day 21, 21.4% lower, RR 0.79, p = 0.51, treatment 11 of 75 (14.7%), control 14 of 75 (18.7%). |
| [Tehrani], 10/30/2020, retrospective, Sweden, Europe, peer-reviewed, 5 authors. | risk of death, 13.4% lower, RR 0.87, p = 0.63, treatment 16 of 65 (24.6%), control 54 of 190 (28.4%). |
| [Texeira], 12/31/2020, retrospective, USA, North America, peer-reviewed, 6 authors. | risk of death, 79.3% higher, RR 1.79, p = 0.10, treatment 17 of 65 (26.2%), control 14 of 96 (14.6%). |
| [Trullàs], 7/14/2020, retrospective, Spain, Europe, preprint, median age 75.0, 8 authors. | risk of death, 35.6% lower, RR 0.64, p = 0.12, treatment 20 of 66 (30.3%), control 16 of 34 (47.1%). |
| [Ubaldo], 2/1/2021, retrospective, Philippines, Asia, peer-reviewed, 3 authors. | risk of death, 18.4% lower, RR 0.82, p = 0.64, treatment 17 of 25 (68.0%), control 5 of 6 (83.3%), COVID-19 positive patients. |
| [Ulrich], 9/23/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, baseline oxygen requirements 63.3%, mean age 66.2, 18 authors. | risk of death, 6.0% higher, RR 1.06, p = 1.00, treatment 7 of 67 (10.4%), control 6 of 61 (9.8%). |
| [van Halem], 11/27/2020, retrospective, Belgium, Europe, peer-reviewed, 10 authors. | risk of death, 31.6% lower, RR 0.68, p = 0.05, treatment 34 of 164 (20.7%), control 47 of 155 (30.3%). |
| [Vernaz], 12/31/2020, retrospective, Switzerland, Europe, peer-reviewed, 15 authors. | risk of death, 15.3% lower, RR 0.85, p = 0.71, treatment 12 of 93 (12.9%), control 16 of 105 (15.2%), HCQ vs. SOC. |
| hospitalization time, 49.0% higher, relative time 1.49, p = 0.002, treatment 93, control 105, HCQ vs. SOC. | |
| Wang], 6/10/2020, retrospective, database analysis, USA, North America, peer-reviewed, 3 authors. | risk of death, 5.8% lower, RR 0.94, p = 0.63, treatment 1866, control 5726, odds ratio converted to relative risk. |
| [Xia], 2/11/2020, retrospective, China, Asia, preprint, 1 author. | risk of no virological cure, 37.5% lower, RR 0.62, p = 0.17, treatment 5 of 10 (50.0%), control 12 of 15 (80.0%). |
| [Yegerov], 1/8/2021, retrospective, Kazakhstan, Asia, peer-reviewed, 8 authors. | risk of death, 95.3% lower, RR 0.05, p = 1.00, treatment 0 of 23 (0.0%), control 20 of 1049 (1.9%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| [Yu (B)], 8/3/2020, retrospective, China, Asia, preprint, median age 62.0, 6 authors | risk of progression to critical, 82.5% lower, RR 0.17, p = 0.05, treatment 1 of 231 (0.4%), control 32 of 1291 (2.5%), baseline critical cohort reported separately in Yu et al.. |
| risk of death, 85.0% lower, RR 0.15, p = 0.02, treatment 1 of 73 (1.4%), control 238 of 2604 (9.1%), HCQ treatment started early vs. non-HCQ. | |
| [Yu (C)], 5/15/2020, retrospective, China, Asia, peer-reviewed, 8 authors. | risk of death, 60.5% lower, RR 0.40, p = 0.002, treatment 9 of 48 (18.8%), control 238 of 502 (47.4%). |
| [Zhong], 3/26/2020, retrospective, China, Asia, preprint, 1 author. | risk of no virological cure at day 10, 80.0% lower, RR 0.20, p < 0.001, treatment 5 of 115 (4.3%), control 17 of 82 (20.7%), adjusted per study. |
| [Águila-Gordo], 11/11/2020, retrospective, Spain, Europe, peer-reviewed, mean age 84.4, 6 authors. | risk of death, 67.0% lower, RR 0.33, p = 0.10, treatment 151 of 346 (43.6%), control 47 of 70 (67.1%), adjusted per study. |
| [Ñamendys-Silva], 10/21/2020, retrospective, database analysis, Mexico, North America, peer-reviewed, mean age 57.3, 18 authors. | risk of death, 32.3% lower, RR 0.68, p = 0.18, treatment 24 of 54 (44.4%), control 42 of 64 (65.6%), HCQ+AZ vs. neither HCQ or CQ. |
| risk of death, 37.1% lower, RR 0.63, p = 0.09, treatment 19 of 46 (41.3%), control 42 of 64 (65.6%), CQ vs. neither HCQ or CQ. | |
| risk of death, 34.5% lower, RR 0.66, p = 0.006, treatment 43 of 100 (43.0%), control 42 of 64 (65.6%), HCQ+AZ or CQ. |
Pre‑Exposure Prophylaxis
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| [Abella], 9/30/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 18 authors. | risk of COVID-19 case, 5.0% lower, RR 0.95, p = 1.00, treatment 4 of 64 (6.2%), control 4 of 61 (6.6%). |
| [Alegiani], 4/15/2021, retrospective, database analysis, Italy, Europe, peer-reviewed, 16 authors. | risk of death, 8.0% higher, RR 1.08, p = 0.64, HCQ vs. other cDMARDs, RR approximated with OR. |
| risk of hospitalization, 18.0% lower, RR 0.82, p = 0.03, HCQ vs. other cDMARDs, RR approximated with OR. | |
| risk of death, 19.0% higher, RR 1.19, p = 0.32, HCQ vs. MTX, RR approximated with OR. | |
| risk of hospitalization, 12.0% lower, RR 0.88, p = 0.17, HCQ vs. MTX, RR approximated with OR. | |
| [Alzahrani], 4/15/2021, retrospective, Saudi Arabia, Middle East, peer-reviewed, 3 authors. | risk of death, 58.8% lower, RR 0.41, p = 1.00, treatment 0 of 14 (0.0%), control 1 of 33 (3.0%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| risk of mechanical ventilation, 81.0% lower, RR 0.19, p = 0.54, treatment 0 of 14 (0.0%), control 3 of 33 (9.1%), continuity correction due to zero event (with reciprocal of the contrasting arm). | |
| risk of COVID-19 severe case, 32.7% lower, RR 0.67, p = 0.70, treatment 2 of 14 (14.3%), control 7 of 33 (21.2%). | |
| [Arleo], 10/27/2020, retrospective, USA, North America, preprint, 5 authors. | risk of death, 50.0% lower, RR 0.50, p = 0.67, treatment 1 of 20 (5.0%), control 5 of 50 (10.0%), all patients. |
| risk of death, 52.0% lower, RR 0.48, p = 0.64, treatment 1 of 10 (10.0%), control 5 of 24 (20.8%), inpatients. | |
| [Bae], 2/20/2021, retrospective, South Korea, Asia, peer-reviewed, 8 authors. | risk of COVID-19 case, 30.3% lower, RR 0.70, p = 0.17, treatment 16 of 743 (2.2%), control 91 of 2698 (3.4%), odds ratio converted to relative risk, PSM. |
| risk of COVID-19 case, 19.5% lower, RR 0.81, p = 0.49, treatment 16 of 743 (2.2%), control 91 of 2698 (3.4%), odds ratio converted to relative risk, PSM, adjusted for region. | |
| risk of COVID-19 case, 30.3% lower, RR 0.70, p = 0.29, treatment 16 of 743 (2.2%), control 91 of 2698 (3.4%), odds ratio converted to relative risk, PSM, adjusted for immunosuppresant use. | |
| risk of COVID-19 case, 40.2% lower, RR 0.60, p = 0.08, odds ratio converted to relative risk, PSM, HCQ >= 6 months. | |
| [Behera], 11/3/2020, retrospective, India, South Asia, peer-reviewed, 13 authors. | risk of COVID-19 case, 27.9% lower, RR 0.72, p = 0.29, treatment 7 of 19 (36.8%), control 179 of 353 (50.7%), adjusted per study, odds ratio converted to relative risk, model 2 conditional logistic regression. |
| risk of COVID-19 case, 26.3% lower, RR 0.74, p = 0.25, treatment 7 of 19 (36.8%), control 179 of 353 (50.7%), odds ratio converted to relative risk, matched pair analysis. | |
| [Bhattacharya], 6/9/2020, retrospective, India, South Asia, preprint, 7 authors. | risk of COVID-19 case, 80.7% lower, RR 0.19, p = 0.001, treatment 4 of 54 (7.4%), control 20 of 52 (38.5%). |
| [Cassione], 5/12/2020, retrospective, Italy, Europe, preprint, survey, median age 52.5, 6 authors. | risk of COVID-19 case, 49.6% higher, RR 1.50, p = 0.59, treatment 10 of 127 (7.9%), control 2 of 38 (5.3%). |
| [Chatterjee], 5/28/2020, retrospective, India, South Asia, peer-reviewed, survey, 11 authors. | risk of COVID-19 case, 66.8% lower, RR 0.33, p < 0.001, treatment 12 of 68 (17.6%), control 206 of 387 (53.2%), full course vs. unused |
| [Cordtz], 12/28/2020, retrospective, Denmark, Europe, peer-reviewed, 10 authors. | risk of hospitalization, 24.0% lower, RR 0.76, p = 0.67, treatment 3 of 2722 (0.1%), control 38 of 26718 (0.1%), adjusted per study, time-dependent exposure model. |
| risk of hospitalization, 55.0% lower, RR 0.45, p = 0.28, treatment 3 of 2722 (0.1%), control 38 of 26718 (0.1%), adjusted per study, time-fixed exposure model. | |
| [de la Iglesia], 9/2/2020, retrospective, database analysis, Spain, Europe, preprint, 17 authors. | risk of hospitalization, 50.0% higher, RR 1.50, p = 1.00, treatment 3 of 687 (0.4%), control 2 of 688 (0.3%). |
| risk of COVID-19 case, 42.6% higher, RR 1.43, p = 0.15, treatment 42 of 648 (6.5%), control 30 of 660 (4.5%), suspected COVID-19. | |
| risk of COVID-19 case, 7.8% lower, RR 0.92, p = 0.84, treatment 12 of 678 (1.8%), control 13 of 677 (1.9%), confirmed COVID-19. | |
| [Desbois], 7/20/2020, retrospective, France, Europe, preprint, mean age 58.8, 13 authors. | risk of COVID-19 case, 16.9% lower, RR 0.83, p = 1.00, treatment 3 of 27 (11.1%), control 23 of 172 (13.4%). |
| [Dev], 3/24/2021, retrospective, India, South Asia, peer-reviewed, 5 authors. | risk of COVID-19 case, 26.0% lower, RR 0.74, p = 0.003, treatment 260, control 499, any number of HCQ doses vs. no HCQ prophylaxis. |
| [Ferreira], 6/29/2020, retrospective, database analysis, Portugal, Europe, peer-reviewed, 3 authors. | risk of COVID-19 case, 47.1% lower, RR 0.53, p < 0.001, adjusted per study, odds ratio converted to relative risk. |
| [Ferri], 8/27/2020, retrospective, Italy, Europe, peer-reviewed, survey, 29 authors. | risk of COVID-19 case, 63.0% lower, RR 0.37, p = 0.01, treatment 9 of 994 (0.9%), control 16 of 647 (2.5%). |
| [Fitzgerald], 2/5/2021, retrospective, USA, North America, preprint, 34 authors. | risk of COVID-19 case, 8.5% lower, RR 0.91, p = 0.52, treatment 65 of 1072 (6.1%), control 200 of 3594 (5.6%), adjusted per study, odds ratio converted to relative risk |
| [Gendebien], 6/25/2020, retrospective, Belgium, Europe, preprint, survey, 9 authors. | risk of COVID-19 case, 3.9% lower, RR 0.96, p = 0.93, treatment 12 of 152 (7.9%), control 6 of 73 (8.2%). |
| Gendelman], 5/5/2020, retrospective, database analysis, Israel, Middle East, peer-reviewed, 5 authors. | risk of COVID-19 case, 8.1% lower, RR 0.92, p = 0.88, treatment 3 of 36 (8.3%), control 1314 of 14484 (9.1%). |
| [Gentry], 9/21/2020, retrospective, database analysis, USA, North America, peer-reviewed, 6 authors. | risk of death, 91.3% lower, RR 0.09, p = 0.10, treatment 0 of 10703 (0.0%), control 7 of 21406 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), COVID-19 mortality within all patients. |
| risk of death, 90.7% lower, RR 0.09, p = 0.19, treatment 0 of 31 (0.0%), control 7 of 78 (9.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), mortality for infected patients. | |
| risk of COVID-19 case, 20.9% lower, RR 0.79, p = 0.27, treatment 31 of 10703 (0.3%), control 78 of 21406 (0.4%), odds ratio converted to relative risk. | |
| [Gianfrancesco], 5/28/2020, retrospective, database analysis, multiple countries, multiple regions, peer-reviewed, 28 authors. | risk of hospitalization, 3.3% lower, RR 0.97, p = 0.82, treatment 58 of 130 (44.6%), control 219 of 470 (46.6%), odds ratio converted to relative risk. |
| [Goenka], 10/24/2020, retrospective, India, South Asia, preprint, 11 authors. | risk of IgG positive, 87.2% lower, RR 0.13, p = 0.03, treatment 1 of 77 (1.3%), control 115 of 885 (13.0%), adjusted per study, odds ratio converted to relative risk. |
| [Grau-Pujol], 9/21/2020, Randomized Controlled Trial, Spain, Europe, preprint, 22 authors. | risk of COVID-19 case, 67.9% lower, RR 0.32, p = 0.47, treatment 0 of 142 (0.0%), control 1 of 127 (0.8%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| [Gönenli], 12/16/2020, retrospective, Turkey, Middle East, preprint, survey, 4 authors. | risk of pneumonia, 29.7% lower, RR 0.70, p = 0.77, treatment 3 of 148 (2.0%), control 12 of 416 (2.9%). |
| risk of COVID-19 case, 18.9% higher, RR 1.19, p = 0.58, treatment 8 of 148 (5.4%), control 20 of 416 (4.8%), odds ratio converted to relative risk. | |
| [Huang], 6/16/2020, retrospective, China, Asia, peer-reviewed, 15 authors. | risk of hospitalization, 80.0% lower, RR 0.20, p < 0.001, treatment 8, control 1247. |
| [Huh], 12/19/2020, retrospective, database analysis, South Korea, Asia, peer-reviewed, 8 authors. | risk of disease progression, 251.0% higher, RR 3.51, p = 0.11, treatment 5 of 8 (62.5%), control 873 of 2797 (31.2%), adjusted per study. |
| risk of COVID-19 case, 6.0% lower, RR 0.94, p = 0.82, treatment 17 of 122 (13.9%), control 7324 of 36600 (20.0%), adjusted per study. | |
| [Huh (B)], 5/4/2020, retrospective, database analysis, South Korea, Asia, preprint, 10 authors. | risk of COVID-19 case, 47.7% higher, RR 1.48, p = 0.09, odds ratio converted to relative risk. |
| [Jung], 12/11/2020, retrospective, South Korea, Asia, peer-reviewed, 6 authors. | risk of death, 59.3% lower, RR 0.41, p = 1.00, treatment 0 of 649 (0.0%), control 1 of 1417 (0.1%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
| risk of COVID-19 case, 13.1% higher, RR 1.13, p = 0.86, treatment 15 of 649 (2.3%), control 31 of 1417 (2.2%), adjusted per study. | |
| [Khurana], 7/24/2020, retrospective, India, South Asia, preprint, survey, 5 authors. | risk of COVID-19 case, 51.0% lower, RR 0.49, p = 0.02, treatment 6 of 22 (27.3%), control 88 of 159 (55.3%), odds ratio converted to relative risk. |
| [Konig], 5/7/2020, retrospective, database analysis, multiple countries, multiple regions, preprint, 11 authors. | risk of hospitalization, 3.0% lower, RR 0.97, p = 0.88, treatment 16 of 29 (55.2%), control 29 of 51 (56.9%). |
| [Laplana], 9/9/2020, retrospective, Spain, Europe, peer-reviewed, survey, 3 authors. | risk of COVID-19 case, 56.0% higher, RR 1.56, p = 0.24, treatment 17 of 319 (5.3%), control 11 of 319 (3.4%). |
| [Macias], 5/16/2020, retrospective, database analysis, Spain, Europe, preprint, 12 authors. | risk of hospitalization, 25.5% lower, RR 0.74, p = 1.00, treatment 1 of 290 (0.3%), control 2 of 432 (0.5%). |
| risk of COVID-19 case, 49.0% higher, RR 1.49, p = 0.53, treatment 5 of 290 (1.7%), control 5 of 432 (1.2%). | |
| [Mathai], 11/6/2020, retrospective, India, South Asia, peer-reviewed, 3 authors. | risk of COVID-19 case, 89.5% lower, RR 0.10, p < 0.001, treatment 10 of 491 (2.0%), control 22 of 113 (19.5%). |
| risk of COVID-19 case, 88.5% lower, RR 0.12, p < 0.001, treatment 5 of 491 (1.0%), control 10 of 113 (8.8%), symptomatic. | |
| [Mitchell], 5/5/2020, retrospective, multiple countries, multiple regions, preprint, 2 authors. | risk of death, 99.0% lower, RR 0.01, p < 0.001. |
| [Pham], 3/2/2021, retrospective, USA, North America, peer-reviewed, 5 authors. | risk of death, 19.7% lower, RR 0.80, p = 0.77, treatment 2 of 14 (14.3%), control 5 of 28 (17.9%), odds ratio converted to relative risk, univariate. |
| risk of ICU admission, 35.5% higher, RR 1.35, p = 0.61, treatment 4 of 14 (28.6%), control 6 of 28 (21.4%), odds ratio converted to relative risk, univariate. | |
| [Rajasingham], 9/21/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 22 authors. | risk of hospitalization, 50.1% lower, RR 0.50, p = 1.00, treatment 1 of 989 (0.1%), control 1 of 494 (0.2%). |
| risk of COVID-19 case, 27.0% lower, RR 0.73, p = 0.12, treatment 58 of 989 (5.9%), control 39 of 494 (7.9%). | |
| [Rangel], 1/10/2021, retrospective, USA, North America, peer-reviewed, 5 authors. | risk of death, 25.1% lower, RR 0.75, p = 0.77, treatment 4 of 50 (8.0%), control 11 of 103 (10.7%), from all patients. |
| risk of hospitalization, 22.2% lower, RR 0.78, p = 0.29, treatment 17 of 50 (34.0%), control 45 of 103 (43.7%). | |
| hospitalization time, 41.2% lower, relative time 0.59, p = 0.12, treatment 21, control 54. | |
| [Rentsch], 9/9/2020, retrospective, database analysis, United Kingdom, Europe, peer-reviewed, 34 authors. | risk of death, 3.0% higher, RR 1.03, p = 0.83, adjusted per study. |
| [Revollo], 11/21/2020, retrospective, Spain, Europe, peer-reviewed, 16 authors. | risk of COVID-19 case, 23.0% lower, RR 0.77, p = 0.52, treatment 16 of 69 (23.2%), control 65 of 418 (15.6%), adjusted per study, PSM risk of PCR+. |
| risk of COVID-19 case, 43.0% higher, RR 1.43, p = 0.42, treatment 17 of 60 (28.3%), control 62 of 404 (15.3%), adjusted per study, PSM risk of IgG+. | |
| [Salvarani], 8/6/2020, retrospective, Italy, Europe, peer-reviewed, 18 authors. | reviewed, 18 authors. Submit Corrections or Updates. risk of COVID-19 case, 6.0% lower, RR 0.94, p = 0.75. |
| [Singer], 8/5/2020, retrospective, database analysis, USA, North America, preprint, 3 authors. | risk of COVID-19 case, 9.0% higher, RR 1.09, p = 0.62, treatment 55 of 10700 (0.5%), control 104 of 22058 (0.5%). |
| [Trefond], 1/27/2021, retrospective, France, Europe, preprint, 21 authors. | risk of death, 16.6% higher, RR 1.17, p = 0.80, treatment 4 of 68 (5.9%), control 12 of 183 (6.6%), adjusted per study, odds ratio converted to relative risk. |
| risk of combined death/ICU, 78.2% higher, RR 1.78, p = 0.21, treatment 8 of 71 (11.3%), control 18 of 191 (9.4%), adjusted per study, odds ratio converted to relative risk. | |
| risk of hospitalization, 44.9% higher, RR 1.45, p = 0.12, treatment 24 of 71 (33.8%), control 53 of 191 (27.7%), adjusted per study, odds ratio converted to relative risk. | |
| [Vivanco-Hidalgo], 3/9/2021, retrospective, Spain, Europe, peer-reviewed, 8 authors. | risk of hospitalization, 46.0% higher, RR 1.46, p = 0.10, treatment 40 of 6746 (0.6%), control 50 of 13492 (0.4%), adjusted per study. |
| risk of COVID-19 case, 8.0% higher, RR 1.08, p = 0.50, treatment 97 of 6746 (1.4%), control 183 of 13492 (1.4%), adjusted per study. | |
| [Zhong (B)], 7/3/2020, retrospective, database analysis, China, Asia, peer-reviewed, 20 authors. | risk of COVID-19 case, 91.0% lower, RR 0.09, p = 0.04, treatment 7 of 16 (43.8%), control 20 of 27 (74.1%), adjusted per study. |
Post‑Exposure Prophylaxis
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| [Barnabas], 12/7/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 30 authors. | risk of hospitalization, 3.7% higher, RR 1.04, p = 1.00, treatment 1 of 407 (0.2%), control 1 of 422 (0.2%). |
| risk of COVID-19 case, 27.0% higher, RR 1.27, p = 0.33, treatment 43 of 353 (12.2%), control 33 of 336 (9.8%), adjusted per study, day 14 symptomatic mITT PCR+ AIM. | |
| risk of COVID-19 case, 23.0% higher, RR 1.23, p = 0.41, treatment 40 of 317 (12.6%), control 32 of 309 (10.4%), adjusted per study, day 14 symptomatic mITT PCR+ IDWeek. | |
| risk of COVID-19 case, 10.0% higher, RR 1.10, p = 0.66, treatment 53 of 353 (15.0%), control 45 of 336 (13.4%), adjusted per study, day 14 PCR+ mITT AIM. | |
| risk of COVID-19 case, 1.0% lower, RR 0.99, p = 0.97, treatment 46 of 317 (14.5%), control 43 of 309 (13.9%), adjusted per study, day 14 PCR+ mITT IDWeek. | |
| risk of COVID-19 case, 19.0% lower, RR 0.81, p = 0.23, treatment 82 of 387 (21.2%), control 99 of 393 (25.2%), adjusted per study, day 14 PCR+ ITT AIM. | |
| [Boulware (B)], 6/3/2020, Randomized Controlled Trial, USA, North America, peer-reviewed, 24 authors. | risk of COVID-19 case, 17.0% lower, RR 0.83, p = 0.35, treatment 49 of 414 (11.8%), control 58 of 407 (14.3%). |
| risk of COVID-19 case, 25.1% lower, RR 0.75, p = 0.22, treatment 32 of 414 (7.7%), control 42 of 407 (10.3%), probable COVID-19 cases. | |
| [Dhibar], 11/6/2020, prospective, India, South Asia, peer-reviewed, 13 authors. | risk of COVID-19 case, 41.0% lower, RR 0.59, p = 0.03, treatment 14 of 132 (10.6%), control 36 of 185 (19.5%), adjusted per study. |
| risk of COVID-19 case, 50.0% lower, RR 0.50, p = 0.04, treatment 10 of 132 (7.6%), control 28 of 185 (15.1%), adjusted per study, PCR+. | |
| risk of symptomatic case, 43.9% lower, RR 0.56, p = 0.21, treatment 6 of 132 (4.5%), control 15 of 185 (8.1%), adjusted per study. | |
| [Mitjà (B)], 7/26/2020, Randomized Controlled Trial, Spain, Europe, peer-reviewed, 12 authors | risk of death, 51.7% lower, RR 0.48, p = 0.27, treatment 4 of 1196 (0.3%), control 9 of 1301 (0.7%), per supplemental appendix table S7, one treatment death was a patient that did not take any study medication, they have been moved to the control group. |
risk of death, 51.7% lower, RR 0.48, p = 0.27, treatment 4 of 1196 (0.3%), control 9 of 1301 (0.7%), per supplemental appendix table S7, one treatment death was a patient that did not take any study medication, they have been moved to the control group. risk of hospitalization, 21.4% lower, RR 0.79, p = 0.59, treatment 13 of 1196 (1.1%), control 18 of 1301 (1.4%), per supplemental appendix table S7, one treatment death was a patient that did not take any study medication, they have been moved to the control group. | |
| baseline pcr- risk of cases, 32.0% lower, RR 0.68, p = 0.27, treatment 29 of 958 (3.0%), control 45 of 1042 (4.3%). | |
| [Polat], 9/30/2020, prospective, Turkey, Middle East, peer-reviewed, 3 authors. | risk of COVID-19 case, 57.0% lower, RR 0.43, p = 0.03, treatment 12 of 138 (8.7%), control 14 of 70 (20.0%). |
| [Seet], 4/14/2021, Cluster Randomized Controlled Trial, Singapore, Asia, peer-reviewed, 15 authors, dosage 400mg day 1, 200mg days 2-42, this trial compares with another treatment – results may be better when compared to placebo. | risk of COVID-19 severe case, 35.1% lower, RR 0.65, p = 0.14, treatment 29 of 432 (6.7%), control 64 of 619 (10.3%). |
| risk of COVID-19 case, 32.0% lower, RR 0.68, p = 0.009, treatment 212 of 432 (49.1%), control 433 of 619 (70.0%), adjusted per study, odds ratio converted to relative risk, model 6. | |
| [Simova (B)], 11/12/2020, retrospective, Bulgaria, Europe, peer-reviewed, 5 authors. | risk of COVID-19 case, 92.7% lower, RR 0.07, p = 0.01, treatment 0 of 156 (0.0%), control 3 of 48 (6.2%), continuity correction due to zero event (with reciprocal of the contrasting arm). |
References
1.Abd-Elsalam et al., American Journal of Tropical Medicine and Hygiene, 10.4269/ajtmh.20-0873, Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study, https://www.ajtmh.org/content/journals/10.4269/ajtmh.20-0873.
2.Abdulrahman et al., medRxiv, doi:10.1101/2020.11.25.20234914, The efficacy and safety of hydroxychloroquine in COVID19 patients : a multicenter national retrospective cohort , https://www.medrxiv.org/content/10.1101/2020.11.25.20234914v1
3.Abella et al., JAMA Internal Medicine, doi:doi:10.1001/jamainternmed.2020.6319, Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers, https://jamanetwork.com/journals/j..ternalmedicine/fullarticle/2771265.
4.Ader et al., medRxiv, doi:10.1101/2021.01.08.20248149 (news 10/6), Antiviral drugs in hospitalized patients with COVID-19 – the DisCoVeRy trial, https://www.medrxiv.org/content/10.1101/2021.01.08.20248149v1.
5.AFP, India backs hydroxychloroquine for virus prevention, https://www.msn.com/en-ph/news/wor..us-prevention/ar-BB14EloP?ocid=st2.
6.AfricaFeeds, Kenya approve the use of Chloroquine to treat COVID-19 patients, https://africafeeds.com/2020/04/01..oquine-to-treat-covid-19-patients/.
7.Africanews, Coronavirus patients on chloroquine heal faster – Senegalese medic, https://www.africanews.com/2020/04..uine-heal-faster-senegalese-medic/.
8.Afrik.com, Edouard Philippe emporté par le Covid, Didier Raoult, l’hydroxychloroquine et le… remdésivir, https://www.afrik.com/edouard-phil..ydroxychloroquine-et-le-remdesivir.
9.Águila-Gordo et al., Revista Española de Geriatría y Gerontología, doi:10.1016/j.regg.2020.09.006, Mortality and associated prognostic factors in elderly and very elderly hospitalized patients with respiratory disease COVID-19, https://www.sciencedirect.com/science/article/pii/S0211139X20301748.
10.Agusti et al., Enfermedades Infecciosas y Microbiología Clínica, doi:10.1016/j.eimc.2020.10.023, Efficacy and safety of hydroxychloroquine in healthcare professionals with mild SARS-CoV-2 infection: prospective, non-randomized trial, https://www.sciencedirect.com/scie../article/abs/pii/S0213005X20304134.
11.Al Arabia, Bahrain among first countries to use Hydroxychloroquine to treat coronavirus, https://english.alarabiya.net/en/N..xychloroquine-to-treat-coronavirus.
12.Al-bab, Covid-19: Algeria and Morocco continue using chloroquine despite concerns, https://al-bab.com/blog/2020/05/co..using-chloroquine-despite-concerns.13.Alamdari et al., Tohoku J. Exp. Med., 2020, 252, 73-84, doi:10.1620/tjem.252.73, Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran, https://www.jstage.jst.go.jp/artic..em/252/1/252_73/_article/-char/ja/.
14.Albani et al., J, Clinical Medicine, doi:10.3390/jcm9092800, Impact of Azithromycin and/or Hydroxychloroquine on Hospital Mortality in COVID-19, https://www.mdpi.com/2077-0383/9/9/2800.
15.Alberici et al., Kidney Int., 98:1, 20-26, July 1, 2020, doi:10.1016/j.kint.2020.04.030 (preprint 5/10), A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection, https://www.kidney-international.o..cle/S0085-2538(20)30508-1/fulltext.
16.Alegiani et al., Rheumatology, doi:10.1093/rheumatology/keab348, Risk of COVID-19 hospitalization and mortality in rheumatic patients treated with hydroxychloroquine or other conventional DMARDs in Italy, https://academic.oup.com/rheumatol..ogy/keab348/6226505?searchresult=1.
17.Alghamdi et al., Antibiotics, doi:10.3390/antibiotics10040365, Clinical Efficacy of Hydroxychloroquine in Patients with COVID-19: Findings from an Observational Comparative Study in Saudi Arabia, https://www.mdpi.com/2079-6382/10/4/365.
18.Almazrou et al., Saudi Pharmaceutical Journal, doi:10.1016/j.jsps.2020.09.019, Comparing the impact of Hydroxychloroquine based regimens and standard treatment on COVID-19 patient outcomes: A retrospective cohort study, https://www.sciencedirect.com/science/article/pii/S1319016420302334.
19.Alqassieh et al., F1000Research, Preprint, Clinical characteristics and predictors of the duration of hospital stay in COVID-19 patients in Jordan, https://f1000research.com/articles/9-1439.
20.Altman, D., BMJ, doi:10.1136/bmj.d2304, How to obtain the P value from a confidence interval, https://www.bmj.com/content/343/bmj.d2304.
21.Altman (B) et al., BMJ, doi:10.1136/bmj.d2090, How to obtain the confidence interval from a P value, https://www.bmj.com/content/343/bmj.d2090.
22.Alzahrani et al., Rheumatology International , doi:10.1007/s00296-021-04857-9, Clinical characteristics and outcome of COVID-19 in patients with rheumatic diseases, https://link.springer.com/article/10.1007/s00296-021-04857-9.
23.Amaravadi et al., medRxiv, doi:10.1101/2021.02.22.21252228, Hydroxychloroquine for SARS-CoV-2 positive patients quarantined at home: The first interim analysis of a remotely conducted randomized clinical trial, https://www.medrxiv.org/content/10.1101/2021.02.22.21252228v1.
24.An et al., medRxiv, doi:10.1101/2020.07.04.20146548, Treatment Response to Hydroxychloroquine and Antibiotics for mild to moderate COVID-19: a retrospective cohort study from South Korea, https://www.medrxiv.org/content/10.1101/2020.07.04.20146548v.
25.Anadolu Agency, Nigeria goes on with hydroxychloroquine clinical trial, https://www.aa.com.tr/en/africa/ni..hloroquine-clinical-trials/1854814.
26.Anadolu Agency (B), Cuba: Early hydroxychloroquine potent against COVID-19, https://www.aa.com.tr/en/americas/..ne-potent-against-covid-19/1905650.
27.Anglemyer et al., Cochrane Database of Systematic Reviews 2014, Issue 4, doi:10.1002/14651858.MR000034.pub2, Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials, https://www.cochranelibrary.com/cd..0.1002/14651858.MR000034.pub2/full.
28.Annie et al., Pharmacotherapy, doi:10.1002/phar.2467, Hydroxychloroquine in hospitalized COVID‐19 patients: Real world experience assessing mortality, https://accpjournals.onlinelibrary.wiley.com/doi/10.1002/phar.2467.
29.Aparisi et al., medRxiv, doi:10.1101/2020.10.06.20207092, Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19, https://www.medrxiv.org/content/10.1101/2020.10.06.20207092v1.
30.Archyde, China approves chloroquine (instead of hydroxychloroquine) against covid-19, https://www.archyde.com/china-appr..droxychloroquine-against-covid-19/.
31.Arleo et al., medRxiv, doi:10.1101/2020.10.26.20219154, Clinical Course and Outcomes of coronavirus disease 2019 (COVID-19) in Rheumatic Disease Patients on Immunosuppression: A case Cohort Study at a Single Center with a Significantly Diverse Population, https://www.medrxiv.org/content/10.1101/2020.10.26.20219154v1.
32.Arshad et al., Int. J. Infect. Dis., July 1 2020, doi:10.1016/j.ijid.2020.06.099, Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19, https://www.ijidonline.com/article/S1201-9712(20)30534-8/fulltext.
33.Ashinyo et al., Pan African Medical Journal, 37:1, doi:10.11604/pamj.supp.2020.37.1.25718, Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study, https://www.panafrican-med-journal.com/content/series/37/1/9/full/.
34.Ashraf et al., medRxiv doi:10.1101/2020.04.20.20072421.t, COVID-19 in Iran, a comprehensive investigation from exposure to treatment outcomes, https://www.researchgate.net/publi..rom_exposure_to_treatment_outcomes.
35.Auld et al., Critical Care Medicine, doi:10.1097/ccm.0000000000004457, ICU and ventilator mortality among critically ill adults with COVID-19, https://journals.lww.com/ccmjourna..ality_Among_Critically_Ill.35.aspx.
36.Awad et al., American Journal of Health-System Pharmacy, doi:10.1093/ajhp/zxab056, Impact of hydroxychloroquine on disease progression and ICU admissions in patients with SARS-CoV-2 infection, https://academic.oup.com/ajhp/adva..e/doi/10.1093/ajhp/zxab056/6144083.
37.Axfors et al., Nature, doi:10.1038/s41467-021-22446-z, Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials, https://www.nature.com/articles/s41467-021-22446-z.
38.Ayerbe et al., Internal and Emergency Medicine, doi:0.1007/s11739-020-02505-x, The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients, https://link.springer.com/article/10.1007/s11739-020-02505-x.
39.Bae et al., Viruses 2021, doi:10.3390/v13020329, Recent Hydroxychloroquine Use Is Not Significantly Associated with Positive PCR Results for SARS-CoV-2: A Nationwide Observational Study in South Korea, https://www.mdpi.com/1999-4915/13/2/329.
40.Barbosa et al., Preprint, Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study, https://www.sefq.es/_pdfs/NEJM_Hydroxychlorquine.pdf.
41.Barnabas et al., Annals of Internal Medicine, doi:10.7326/M20-6519, Hydroxychloroquine for Post-exposure Prophylaxis to Prevent Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Trial, https://www.acpjournals.org/doi/10.7326/M20-6519.
42.Barron’s, Hydroxychloroquine: A Drug Dividing The World, https://www.barrons.com/news/hydro..rug-dividing-the-world-01591006809.
43.Barron’s (B), Amid Global Controversy, Greece Moves Forward With Chloroquine, https://www.barrons.com/news/amid-..rward-with-chloroquine-01591781707.
44.Barry et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.03.058, Clinical Characteristics and Outcomes of Hospitalized COVID-19 Patients in a MERS-CoV Referral Hospital during the Peak of the Pandemic, https://www.sciencedirect.com/science/article/pii/S1201971221002769.
45.BBC, Coronavirus: How Turkey took control of Covid-19 emergency, https://www.bbc.com/news/world-europe-52831017.
46.Behera et al., PLoS ONE, doi:10.1371/journal.pone.0247163 (preprint 11/3), Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0247163.
47.Belayneh, A., Off-Label Use of Chloroquine and Hydroxychloroquine for COVID-19 Treatment in Africa Against WHO Recommendation, https://www.dovepress.com/off-labe..eer-reviewed-fulltext-article-RRTM.
48.Beltran-Gonzalez et al., medRxiv, doi:10.1101/2021.02.18.21252037, Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial, https://www.medrxiv.org/content/10.1101/2021.02.18.21252037v1.
49.Beltran-Gonzalez (B) et al., medRxiv, doi:10.1101/2021.02.18.21252037, Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial, https://www.medrxiv.org/content/10.1101/2021.02.18.21252037v1.
50.Berenguer et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2020.07.024, Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain, https://www.clinicalmicrobiologyan..cle/S1198-743X(20)30431-6/fulltext.
51.Bernabeu-Wittel et al., J. Gerontol. A Biol. Sci. Med. Sci., doi:10.1093/gerona/glaa192, Effectiveness of a On-Site Medicalization Program for Nursing Homes with COVID-19 Outbreaks, https://academic.oup.com/biomedger..doi/10.1093/gerona/glaa192/5879759.
52.Bernaola et al., medRxiv, doi:10.1101/2020.07.17.20155960, Observational Study of the Efficiency of Treatments in Patients Hospitalized with Covid-19 in Madrid, https://www.medrxiv.org/content/10.1101/2020.07.17.20155960v1.
53.Berry et al., SSRN, Berry, doi:10.2139/ssrn.3707327., Unfavorable Hydroxychloroquine COVID-19 Research Associated with Authors Having a History of Political Party Donations, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3707327.
54.Bhattacharya et al., medRxix, doi:10.1101/2020.06.09.20116806, Pre exposure Hydroxychloroquine use is associated with reduced COVID19 risk in healthcare workers, https://www.medrxiv.org/content/10.1101/2020.06.09.20116806v1.
55.Bianet, Turkey begins distributing hydroxychloroquine to homes in capital city amid bed shortage, https://bianet.org/english/health/..-in-capital-city-amid-bed-shortage.
56.Bielza et al., Journal of the American Medical Directors Association, doi:10.1016/j.jamda.2020.12.003, Clinical characteristics, frailty and mortality of residents with COVID-19 in nursing homes of a region of Madrid, https://www.sciencedirect.com/science/article/pii/S1525861020310525.
57.Boari et al, Biosci. Rep., doi:10.1042/BSR20203455, Prognostic factors and predictors of outcome in patients with COVID-19 and related pneumonia: a retrospective cohort study, https://portlandpress.com/bioscire..cle/doi/10.1042/BSR20203455/226985.
58.Borba et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2020.8857, Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study), https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2765499.
59.Boulware, D., Comments regarding paper rejection, https://twitter.com/boulware_dr/status/1311331372884205570.
60.Boulware (B) et al., NEJM, June 3 2020, doi:10.1056/NEJMoa2016638, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, https://www.nejm.org/doi/full/10.1056/NEJMoa2016638.
61.Bousquet et al., Aging, 12:12, 11306-11313, doi:10.18632/aging.103583, ADL-dependency, D-Dimers, LDH and absence of anticoagulation are independently associated with one-month mortality in older inpatients with Covid-19, https://www.aging-us.com/article/103583/text.
62.Budhiraja et al., medRxiv, doi:10.1101/2020.11.16.20232223, Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience, https://www.medrxiv.org/content/10.1101/2020.11.16.20232223v1.
63.Cadegiani et al., medRxiv, doi:10.1101/2020.10.31.20223883, Early COVID-19 Therapy with Azithromycin Plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Reduced Symptoms Compared to Known Outcomes in Untreated Patients, https://www.medrxiv.org/content/10.1101/2020.10.31.20223883v1.
64.Cangiano et al., Aging, doi:10.18632/aging.202307, Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests, https://www.aging-us.com/article/202307/text.
65.Capsoni et al., Research Square, doi:10.21203/rs.3.rs-113418/v1, CPAP Treatment In COVID-19 Patients: A Retrospective Observational Study In The Emergency Department, https://www.researchsquare.com/article/rs-113418/v1.
66.Cassione et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217717, COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine, https://ard.bmj.com/content/early/..05/23/annrheumdis-2020-217717.info.
67.Catteau et al., Int. J. Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106144, Low-dose Hydroxychloroquine Therapy and Mortality in Hospitalized Patients with COVID-19: A Nationwide Observational Study of 8075 Participants, https://www.sciencedirect.com/scie../article/abs/pii/S0924857920303423.
68.Cavalcanti et al., NEJM, July 23, 2020, doi:10.1056/NEJMoa2019014, Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, https://www.nejm.org/doi/full/10.1056/NEJMoa2019014.
69.CBS News, Turkey claims success treating virus with drug touted by Trump, https://www.msn.com/en-au/news/wor..h-drug-touted-by-trump/ar-BB13oMXS.
70.Challenge, Coronavirus : ce que le Maroc a réussi, https://www.challenge.ma/coronavirus-ce-que-le-maroc-a-reussi-144484/.
71.Chari et al., Blood, doi:10.1182/blood.2020008150, Clinical features associated with COVID-19 outcome in multiple myeloma: first results from the International Myeloma Society data set, https://www.sciencedirect.com/science/article/pii/S0006497120839044.
72.Chatterjee et al., Indian J. Med. Res., June 20, 2020, doi:10.4103/ijmr.IJMR_2234_20, Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19, https://www.ijmr.org.in/article.as..ge=459;epage=467;aulast=Chatterjee.
73.Chen et al., medRxiv, doi:10.1101/2020.06.19.20136093, Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study, https://www.medrxiv.org/content/10.1101/2020.06.19.20136093v1.
74.Chen (B) et al., PLoS ONE, doi:10.1371/journal.pone.0242763, A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19), https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0242763.
75.Chen (C) et al., PLoS ONE, doi:10.1371/journal.pone.0242763, A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19), https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0242763.
76.Chen (D) et al., medRxiv doi:10.1101/2020.03.22.20040758, Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3.
77.Chen (E) et al., J. Zhejiang University (Med Sci), doi:10.3785/j.issn.1008-9292.2020.03.03, A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19), http://www.zjujournals.com/med/EN/..cleFile.do?attachType=PDF&id=41137.
78.Choi et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.10.062, Comparison of antiviral effect for mild-to-moderate COVID-19 cases between lopinavir/ritonavir versus hydroxychloroquine: A nationwide propensity score-matched cohort study, https://www.sciencedirect.com/science/article/pii/S1201971220322669.
79.Coll et al., American Journal of Transplantation, doi:10.1111/ajt.16369, Covid‐19 in transplant recipients: the spanish experience, https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.16369.
80.Concato et al., NEJM, 342:1887-1892, doi:10.1056/NEJM200006223422507, https://www.nejm.org/doi/full/10.1056/nejm200006223422507.
81.Cordtz et al., Rheumatology, doi:10.1093/rheumatology/keaa897, Incidence and severeness of COVID-19 hospitalisation in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark, https://academic.oup.com/rheumatol…1093/rheumatology/keaa897/6053804.
82.Cravedi et al., American Journal of Transplantation, doi:10.1111/ajt.16185, COVID‐19 and kidney transplantation: Results from the TANGO International Transplant Consortium, https://onlinelibrary.wiley.com/doi/full/10.1111/ajt.16185.
83.D’Arminio Monforte et al., Int. J. Infectious Diseases, doi:10.1016/j.ijid.2020.07.056, Effectiveness of Hydroxychloroquine in COVID-19 disease: A done and dusted situation?, https://www.ijidonline.com/article/S1201-9712(20)30600-7/fulltext.
84.Davido et al., Int. J. Antimicrobial Agents, 2020, doi:10.1016/j.ijantimicag.2020.106129, Impact of medical care including anti-infective agents use on the prognosis of COVID-19 hospitalized patients over time, https://www.sciencedirect.com/science/article/pii/S0924857920303125.
85.de la Iglesia et al., medRxiv, doi:10.1101/2020.08.31.20185314, Hydroxicloroquine for pre-exposure prophyylaxis for SARS-CoV-2, https://www.medrxiv.org/content/10.1101/2020.08.31.20185314v1.
86.Deaton et al., Social Science & Medicine, 210, doi:10.1016/j.socscimed.2017.12.005, Understanding and misunderstanding randomized controlled trials, https://www.sciencedirect.com/science/article/pii/S0277953617307359.
87.Deng, H., PyMeta, Python module for meta-analysis, http://www.pymeta.com/.
88.Derwand et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106214 (preprint 7/3), COVID-19 Outpatients – Early Risk-Stratified Treatment with Zinc Plus Low Dose Hydroxychloroquine and Azithromycin: A Retrospective Case Series Study, https://www.sciencedirect.com/science/article/pii/S0924857920304258.
89.Desbois et al., Research Square, doi:10.21203/rs.3.rs-41653/v1, Prevalence and clinical features of COVID-19 in a large cohort of 199 patients with sarcoidosis, https://www.researchsquare.com/article/rs-41653/v1.
90.Dev et al., Transactions of The Royal Society of Tropical Medicine and Hygiene, doi:10.1093/trstmh/trab047, Risk factors and frequency of COVID-19 among healthcare workers at a tertiary care centre in India: a case–control study, https://academic.oup.com/trstmh/ad..doi/10.1093/trstmh/trab047/6186057.
91.Dhibar et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106224, Post Exposure Prophylaxis with Hydroxychloroquine (HCQ) for the Prevention of COVID-19, a Myth or a Reality? The PEP-CQ Study, https://www.sciencedirect.com/science/article/pii/S0924857920304350.
92.Di Castelnuovo et al., European J. Internal Medicine, doi:10.1016/j.ejim.2020.08.019, Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study, https://www.sciencedirect.com/scie../article/abs/pii/S0953620520303356.
93.Dr. Goldin, Summary of HCQ usage in India from an MD in India, https://www.facebook.com/groups/hy..oquine/permalink/2367454293560817/.
94.Dubee et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2021.03.005 (preprint 10/21), Hydroxychloroquine in mild-to-moderate COVID-19: a placebo-controlled double blind trial, https://www.sciencedirect.com/science/article/pii/S1198743X21001403.
95.Dubernet et al., J. Global Antimicrobial Resistance, doi:10.1016/j.jgar.2020.08.001, A comprehensive strategy for the early treatment of COVID-19 with azithromycin/hydroxychloroquine and/or corticosteroids: results of a retrospective observational study in the French overseas department of Reunion Island, https://www.sciencedirect.com/science/article/pii/S221371652030206X.
96.Efecto Cocuyo, Venezuela empieza a usar la cloroquina para tratar COVID-19, anuncia Jorge Rodríguez, https://efectococuyo.com/coronavir..-covid-19-anuncia-jorge-rodriguez/.
97.Esper et al., Prevent Senior Institute, São Paulo, Brazil, Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine, https://www.dropbox.com/s/5qm58cd4..20journal%20manuscript%20final.pdf.
98.Expats.cz, Czech Health Ministry permits temporary use of hydroxychloroquine to treat COVID-19, https://news.expats.cz/weekly-czec..ne-in-hospitals-to-treat-covid-19/.
99.Face 2 Face Africa, Djibouti, others warned about chloroquine despite big COVID-19 recoveries, https://face2faceafrica.com/articl..ne-despite-big-covid-19-recoveries.
100.Faíco-Filho et al., Braz J Microbiol, doi:10.1007/s42770-020-00395-x (preprint 6/21), No benefit of hydroxychloroquine on SARS-CoV-2 viral load reduction in non-critical hospitalized patients with COVID-19, https://link.springer.com/article/10.1007/s42770-020-00395-x.
101.Falcone et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa563, Role of low-molecular weight heparin in hospitalized patients with SARS-CoV-2 pneumonia: a prospective observational study, https://academic.oup.com/ofid/adva..e/doi/10.1093/ofid/ofaa563/5992463.
102.Ferreira et al., J. Medical Virology, July 9, 2020, doi:10.1002/jmv.26286 (preprint 6/29), Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection, https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.26286.
103.Ferri at al., Clinical Rheumatology, doi:0.1007/s10067-020-05334-7, COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series, https://link.springer.com/article/10.1007/s10067-020-05334-7.
104.Filipova et al., Health Science Journal, Is there a Correlation between Changes in Hydroxychloroquine Use and Mortality Rates from COVID-19?, https://www.hsj.gr/medicine/is-the..nd-mortalityrates-from-covid19.pdf.
105.Fitzgerald et al., medRxiv, doi:10.1101/2021.02.03.21251069, Risk Factors for Infection and Health Impacts of the COVID-19 Pandemic in People with Autoimmune Diseases, https://www.medrxiv.org/content/10.1101/2021.02.03.21251069v1.
106.Fonseca et al., Travel Medicine and Infectious Disease, doi:10.1016/j.tmaid.2020.101906, Risk of Hospitalization for Covid-19 Outpatients Treated with Various Drug Regimens in Brazil: Comparative Analysis, https://www.sciencedirect.com/scie../article/abs/pii/S1477893920304026.
107.Fontana et al., Clinical Kidney Journal, 13:3, 334–339, doi:10.1093/ckj/sfaa084, SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience, https://academic.oup.com/ckj/article/13/3/334/5860798.
108.France 24, Covid-19: In Cameroon, chloroquine therapy hailed by French expert becomes state protocol, https://www.france24.com/en/202005..ench-expert-becomes-state-protocol.
109.France 24 (B), Covid-19 : au Cameroun, la méthode Raoult érigée en protocole d’État, https://www.france24.com/fr/202005..ig%C3%A9e-en-protocole-d-%C3%A9tat.
110.Franceinfo, Ces pays africains qui ont décidé de continuer à soigner le Covid-19 avec l’hydroxychloroquine, https://www.francetvinfo.fr/monde/..-l-hydroxychloroquine_3983239.html.
111.Fried et al., Clinical Infectious Disease, doi:10.1093/cid/ciaa1268, Patient Characteristics and Outcomes of 11,721 Patients with COVID19 Hospitalized Across the United States, https://academic.oup.com/cid/advan..e/doi/10.1093/cid/ciaa1268/5898276.
112.Frontera et al., Research Square, doi:10.21203/rs.3.rs-94509/v1, Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study, https://www.researchsquare.com/article/rs-94509/v1.
113.Garcia-Albeniz et al., medRxiv, doi:10.1101/2020.09.29.20203869, Brief communication: A meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19, https://www.medrxiv.org/content/10.1101/2020.09.29.20203869v2.
114.Gautret et al., Int. J. of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.105949 (preprint 3/17), Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, https://www.sciencedirect.com/scie../article/abs/pii/S0924857920300996.
115.Geleris et al., NEJM, May 7, 2020, doi:10.1056/NEJMoa2012410, Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19, https://www.nejm.org/doi/full/10.1056/NEJMoa2012410.
116.Gendebien et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-218244, Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments, https://ard.bmj.com/content/early/2020/06/25/annrheumdis-2020-218244.
117.Gendelman et al., Autoimmunity Reviews, 19:7, July 2020, doi:10.1016/j.autrev.2020.102566, Continuous Hydroxychloroquine or Colchicine Therapy Does Not Prevent Infection With SARS-CoV-2: Insights From a Large Healthcare Database Analysis, https://www.sciencedirect.com/science/article/pii/S1568997220301282.
118.Gentry et al., Lancet Rheumatology, doi:10.1016/S2665-9913(20)30305-2, Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study, https://www.thelancet.com/journals../PIIS2665-9913(20)30305-2/fulltext.
119.Gianfrancesco et al., Annals of the Rheumatic Diseases, 79:7, 859-866, doi:10.1136/annrheumdis-2020-217871, Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry, https://europepmc.org/article/med/32471903.
120.Global Times, Chinese medical expert decorated by Djibouti for COVID-19 prevention, https://www.globaltimes.cn/content/1189839.shtml.
121.Goenka et al., SSRN, doi:10.2139/ssrn.3689618, Seroprevalence of COVID-19 Amongst Health Care Workers in a Tertiary Care Hospital of a Metropolitan City from India, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3689618.
122.Goldman et al., NEJM, doi:10.1056/NEJMoa2015301, Remdesivir for 5 or 10 Days in Patients with Severe Covid-19, https://www.nejm.org/doi/10.1056/NEJMoa2015301.
123.Gönenli et al., Research Square, doi:0.21203/rs.3.rs-107937/v1, Prophylactic use of Hydroxychloroquine among Physicians working in Pandemic Hospitals, https://www.researchsquare.com/article/rs-107937/v1.
124.Gonzalez et al., medRxiv, doi:10.1101/2020.08.18.20172874, The Prognostic Value of Eosinophil Recovery in COVID-19: A Multicentre, Retrospective Cohort Study on Patients Hospitalised in Spanish Hospitals, https://www.medrxiv.org/content/10.1101/2020.08.18.20172874v1.
125.Government of China, 关于印发新型冠状病毒肺炎诊疗方案(试行第八版)的通知, http://www.nhc.gov.cn/yzygj/s7653p..df12bd4b46e5bd28ca7f9a7f5e5a.shtml.
126.Government of India, The caregiver and all close contacts of such cases should take HCQ prophylaxis, https://www.mohfw.gov.in/pdf/RevisedHomeIsolationGuidelines.pdf.
127.Government of Venezuela, THERAPEUTIC MANAGEMENT GUIDE FOR COVID-19 PATIENTS AND CONTACTS, http://www.mpps.gob.ve/index.php/sistemas/descargas.
128.Grau-Pujol et al., Research Square, doi:10.21203/rs.3.rs-72132/v1, Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: initial results of a double-blind, placebo-controlled randomized clinical trial, https://www.researchsquare.com/article/rs-350749/v1.
129.Guérin et al., Asian J. Medicine and Health, July 15, 2020, doi:10.9734/ajmah/2020/v18i730224 (preprint 5/31), Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19, https://www.journalajmah.com/index.php/AJMAH/article/view/30224.
130.Guglielmetti et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2020.11.012, Severe COVID-19 pneumonia in Piacenza, Italy – a cohort study of the first pandemic wave, https://www.sciencedirect.com/science/article/pii/S1876034120307516.
131.Guisado-Vasco, Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: A retrospective observational study (COQUIMA cohort), https://www.sciencedirect.com/science/article/pii/S2589537020303357.
132.Guisado-Vasco (B), Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: A retrospective observational study (COQUIMA cohort), https://www.sciencedirect.com/science/article/pii/S2589537020303357.
133.GulfInsider, Coronavirus: Bahrain’s Therapeutic Medication Proved Effective, https://www.gulf-insider.com/coron..eutic-medication-proved-effective/.
134.Güner et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2020.12.017, Comparing ICU Admission Rates of Mild/Moderate COVID-19 Patients Treated with Hydroxychloroquine, Favipiravir, and Hydroxychloroquine plus Favipiravir, https://www.sciencedirect.com/science/article/pii/S1876034120307735.
135.Gupta et al., JAMA Intern. Med., doi:10.1001/jamainternmed.2020.3596, Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US, https://jamanetwork.com/journals/j..ternalmedicine/fullarticle/2768602.
136.Heberto et al., IJC Heart & Vasculature, doi:10.1016/j.ijcha.2020.100638, Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19), https://www.sciencedirect.com/science/article/pii/S2352906720303365.
137.Heras et al., European Geriatric Medicine, doi:10.1007/s41999-020-00432-w (preprint 9/2), COVID-19 mortality risk factors in older people in a long-term care center, https://link.springer.com/article/10.1007/s41999-020-00432-w.
138.Hernandez-Cardenas et al., medRxiv, doi:10.1101/2021.02.01.21250371, Hydroxychloroquine for the treatment of severe respiratory infection by COVID-19: a randomized controlled trial, https://www.medrxiv.org/content/10.1101/2021.02.01.21250371v1.
139.Hong et al., Infect. Chemother., 2020, doi:10.3947/ic.2020.52.e43, Early Hydroxychloroquine Administration for Rapid Severe Acute Respiratory Syndrome Coronavirus 2 Eradication, https://icjournal.org/DOIx.php?id=10.3947/ic.2020.52.3.396.
140.Hraiech et al., Ann. Intensive Care, doi:10.1186/s13613-020-00678-4, Lack of viral clearance by the combination of hydroxychloroquine and azithromycin or lopinavir and ritonavir in SARS-CoV-2-related acute respiratory distress syndrome, https://annalsofintensivecare.spri..rticles/10.1186/s13613-020-00678-4.
141.Huang et al., Annals of the Rheumatic Diseases 2020:79, 1163-1169, doi:10.1136/annrheumdis-2020-217425, Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study, https://ard.bmj.com/content/79/9/1163.
142.Huang (B) et al., National Science Review, nwaa113, doi:10.1093/nsr/nwaa113, Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19, https://academic.oup.com/nsr/advan..le/doi/10.1093/nsr/nwaa113/5848167.
143.Huang (C) et al., National Science Review, nwaa113, doi:10.1093/nsr/nwaa113, Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19, https://academic.oup.com/nsr/advan..le/doi/10.1093/nsr/nwaa113/5848167.
144.Huang (D) et al., Journal of Molecular Cell Biology, Volume 12, Issue 4, April 2020, 322–325, doi:10.1093/jmcb/mjaa014, Treating COVID-19 with Chloroquine, https://academic.oup.com/jmcb/article/12/4/322/5814655.
145.Huh et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.12.041, Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea, https://www.sciencedirect.com/science/article/pii/S1201971220325650.
146.Huh (B) et al., medRxiv, doi:10.1101/2020.05.04.20089904, Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea, https://www.medrxiv.org/content/10.1101/2020.05.04.20089904v2.
147.IHU Marseille, Meta-analysis on chloroquine derivatives and COVID-19 mortality, https://www.mediterranee-infection..9-mortality-october20-2020-update/.
148.Ip et al., BMC Infectious Diseases, doi:10.1186/s12879-021-05773-w (preprint 8/25), Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study, https://bmcinfectdis.biomedcentral..rticles/10.1186/s12879-021-05773-w.
149.Ip (B) et al., PLoS ONE, doi:10.1371/journal.pone.0237693, Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients – An Observational Study, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0237693.
150.Izoulet M., SSRN, doi:10.2139/ssrn.3575899, Countries which Primarily Use Antimalarial Drugs As COVID-19 Treatment See Slower Dynamic of Daily Deaths, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3575899.
151.Johnston et al., EClinicalMedicine, doi:10.1016/j.eclinm.2021.100773 (preprint 12/9), Hydroxychloroquine with or Without Azithromycin for Treatment of Early SARS-CoV-2 Infection Among High-Risk Outpatient Adults: A Randomized Clinical Trial, https://www.thelancet.com/journals../PIIS2589-5370(21)00053-5/fulltext.
152.Jung et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2020.12.003, Effect of hydroxychloroquine pre-exposure on infection with SARS-CoV-2 in rheumatic disease patients: A population-based cohort study, https://www.sciencedirect.com/science/article/pii/S1198743X20307527.
153.Kalligeros et al., Journal of Global Antimicrobial Resistance, doi:10.1016/j.jgar.2020.07.018, Hydroxychloroquine use in hospitalised patients with COVID-19: An observational matched cohort study, https://www.sciencedirect.com/science/article/pii/S2213716520301934.
154.Kamran et al., medRxiv, doi:10.1101/2020.07.30.20165365, Clearing the fog: Is HCQ effective in reducing COVID-19 progression: A randomized controlled trial, https://www.medrxiv.org/content/10.1101/2020.07.30.20165365v1.
155.Kelly et al., British Journal of Clinical Pharmacology, doi:10.1111/bcp.14482, Clinical outcomes and adverse events in patients hospitalised with COVID‐19, treated with off‐label hydroxychloroquine and azithromycin, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bcp.14482.
156.Khurana et al., medRxiv, doi:10.1101/2020.07.21.20159301, Prevalence and clinical correlates of COVID-19 outbreak among healthcare workers in a tertiary level hospital, https://www.medrxiv.org/content/10.1101/2020.07.21.20159301v1.
157.Kim et al., medRxiv, doi:10.1101/2020.05.13.20094193, Treatment Response to Hydroxychloroquine, Lopinavir/Ritonavir, and Antibiotics for Moderate COVID 19: A First Report on the Pharmacological Outcomes from South Korea, https://www.medrxiv.org/content/10..20.05.13.20094193v1?versioned=true.
158.Kirenga et al., BMJ Open Respiratory Research, doi:10.1136/bmjresp-2020-000646, Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda, https://bmjopenrespres.bmj.com/content/7/1/e000646.
159.Komissarov et al., medRxiv, doi:10.1101/2020.06.30.20143289, Hydroxychloroquine has no effect on SARS-CoV-2 load in nasopharynx of patients with mild form of COVID-19, https://www.medrxiv.org/content/10.1101/2020.06.30.20143289v1.
160.Konig et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217690, Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19, https://ard.bmj.com/content/early/2020/05/20/annrheumdis-2020-217690.
161.Kuderer et al., Lancet, June 20, 2020, doi:10.1016/S0140-6736(20)31187-9 (preprint 5/28), Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study, https://www.thelancet.com/journals../PIIS0140-6736(20)31187-9/fulltext.
162.Ladapo et al., medRxiv, doi:10.1101/2020.09.30.20204693, Randomized Controlled Trials of Early Ambulatory Hydroxychloroquine in the Prevention of COVID-19 Infection, Hospitalization, and Death: Meta-Analysis, https://www.medrxiv.org/content/10.1101/2020.09.30.20204693v1.
163.Lagier et al., Travel Med. Infect. Dis. 101791, Jun 25, 2020, doi:10.1016/j.tmaid.2020.101791, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis, https://www.sciencedirect.com/science/article/pii/S1477893920302817.
164.Lamback et al., The Brazilian Journal of Infectious Diseases, doi:10.1016/j.bjid.2021.101549, Hydroxychloroquine with azithromycin in patients hospitalized for mild and moderate COVID-19, https://www.sciencedirect.com/science/article/pii/S141386702100012X.
165.Lambermont et al., Critical Care Explorations, doi:10.1097/CCE.0000000000000305, Predictors of Mortality and Effect of Drug Therapies in Mechanically Ventilated Patients With Coronavirus Disease 2019: A Multicenter Cohort Study, https://journals.lww.com/ccejourna..rtality_and_Effect_of_Drug.10.aspx.
166.Lammers et al., Int. J. Infectious Diseases, doi:10.1016/j.ijid.2020.09.1460, Early hydroxychloroquine but not chloroquine use reduces ICU admission in COVID-19 patients, https://www.sciencedirect.com/science/article/pii/S1201971220321755.
167.Lano et al., Clinical Kidney Journal, 13:5, October 2020, 878–888, doi:10.1093/ckj/sfaa199, Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort, https://academic.oup.com/ckj/article/13/5/878/5934808.
168.Laplana et al., PLOS ONE, doi:10.1371/journal.pone.0243598, Lack of protective effect of chloroquine derivatives on COVID-19 disease in a Spanish sample of chronically treated patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0243598.
169.Lauriola et al., Clinical and Translational Science, doi:10.1111/cts.12860, Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in COVID‐19 patients, https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1111/cts.12860.
170.Le Nouvel Afrik, Covid-19 : pourquoi les Marocains décèdent plus en Europe qu’au Maroc, https://www.afrik.com/covid-19-pou..ecedent-plus-en-europe-qu-au-maroc.
171.Lecronier et al., Critical Care, 24:418, 2020, doi:10.1186/s13054-020-03117-9, Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis, https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03117-9.
172.Lee et al., Arch Intern Med., 2011, 171:1, 18-22, doi:10.1001/archinternmed.2010.482, Analysis of Overall Level of Evidence Behind Infectious Diseases Society of America Practice Guidelines, https://jamanetwork.com/journals/j..nternalmedicine/fullarticle/226373.
173.Li et al., Science China Life Sciences, doi:10.1007/s11427-020-1871-4, Evaluation of the efficacy and safety of hydroxychloroquine in comparison with chloroquine in moderate and severe patients with COVID-19, https://link.springer.com/article/10.1007/s11427-020-1871-4.
174.Li (B) et al., Research Square, doi:10.21203/rs.3.rs-119202/v1, Treatment of COVID-19 patients with hydroxychloroquine or chloroquine: A retrospective analysis, https://www.researchsquare.com/article/rs-119202/v1.
175.LifeSiteNews, Doctors insist this cheap, safe drug is “key to preventing huge loss of life” from Wuhan virus, https://www.lifesitenews.com/news/..huge-loss-of-life-from-covid-virus.
176.López et al., Annals of Pediatrics, doi:10.1016/j.anpedi.2020.10.017 , Telemedicine follow-ups for COVID-19: experience in a tertiary hospital, https://www.sciencedirect.com/science/article/pii/S1695403320304768.
177.Lora-Tamayo et al., J. Infection, doi:10.1016/j.jinf.2021.02.011, Early Lopinavir/ritonavir does not reduce mortality in COVID-19 patients: results of a large multicenter study, https://www.sciencedirect.com/science/article/pii/S0163445321000773.
178.Lotfy et al., Turk. Thorac. J., doi:10.5152/TurkThoracJ.2021.20180, Use of Hydroxychloroquine in Patients with COVID-19: A Retrospective Observational Study, https://turkthoracj.org/en/use-of-..pective-observational-study-131729.
179.Luo et al., Annals of Oncology, 31:10, 1386-1396, doi:10.1016/j.annonc.2020.06.007, COVID-19 in patients with lung cancer, https://www.annalsofoncology.org/a..cle/S0923-7534(20)39894-X/fulltext.
180.Ly et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106219 (preprint 8/21), Pattern of SARS-CoV-2 infection among dependant elderly residents living in retirement homes in Marseille, France, March-June 2020, https://www.sciencedirect.com/scie../article/abs/pii/S0924857920304301.
181.Lyngbakken et al., Nature Communications, doi:10.1038/s41467-020-19056-6, A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics, https://www.nature.com/articles/s41467-020-19056-6.
182.Macias et al., medRxiv, 10.1101/2020.05.16.20104141, Similar incidence of Coronavirus Disease 2019 (COVID-19) in patients with rheumatic diseases with and without hydroxychloroquine therapy, https://www.medrxiv.org/content/10.1101/2020.05.16.20104141v1.
183.Magagnoli et al., Med (2020), doi:10.1016/j.medj.2020.06.001 (preprint 4/21), Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, https://www.sciencedirect.com/science/article/pii/S2666634020300064.
184.Mahévas et al., BMJ 2020, 369, doi: https://doi.org/10.1136/bmj.m1844, Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data, https://www.bmj.com/content/369/bmj.m1844.
185.Maldonado et al., Nefrología, doi:10.1016/j.nefro.2020.09.002, COVID-19 incidence and outcomes in a home dialysis unit in Madrid (Spain) at the height of the pandemic, https://www.sciencedirect.com/science/article/pii/S0211699520301661.
186.Mallat et al., Medicine (Baltimore), doi:10.1097/MD.0000000000023720 (preprint 5/2), Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: A retrospective study, https://journals.lww.com/md-journa..sociated_with_slower_viral.34.aspx.
187.Martin-Vicente et al., medRxiv, doi:10.1101/2021.03.08.21253121, Absent or insufficient anti-SARS-CoV-2 S antibodies at ICU admission are associated to higher viral loads in plasma, antigenemia and mortality in COVID-19 patients, https://www.medrxiv.org/content/10.1101/2021.03.08.21253121v1.
188.Martinez-Lopez et al., Blood Cancer Journal, doi:10.1038/s41408-020-00372-5, Multiple Myeloma and SARS-CoV-2 Infection: Clinical Characteristics and Prognostic Factors of Inpatient Mortality, https://www.nature.com/articles/s41408-020-00372-5.
189.Matangila et al., PLoS ONE, doi:10.1371/journal.pone.0244272, Clinical characteristics of COVID-19 patients hospitalized at Clinique Ngaliema, a public hospital in Kinshasa, in the Democratic Republic of Congo: A retrospective cohort study, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0244272.
190.Mathai et al., J. Marine Medical Society, doi:10.4103/jmms.jmms_115_20, Hydroxychloroquine as pre-exposure prophylaxis against COVID-19 in health-care workers: A single-center experience, https://www.marinemedicalsociety.in/preprintarticle.asp?id=300159.
191.McGrail et al., medRxiv, doi:10.1101/2020.07.17.20156521, COVID-19 Case Series at UnityPoint Health St. Luke’s Hospital in Cedar Rapids, IA, https://www.medrxiv.org/content/10.1101/2020.07.17.20156521v1.
192.McLean et al., Open Forum Infect. Dis. September 2015, 2:3, doi:10.1093/ofid/ofv100, Impact of Late Oseltamivir Treatment on Influenza Symptoms in the Outpatient Setting: Results of a Randomized Trial, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4525010/.
193.Medical World Nigeria, Chloroquine potent for COVID-19 prevention, says NAFDAC, https://medicalworldnigeria.com/po..9-Prevention-Says-NAFDAC?pid=45479.
194.Medical Xpress, Senegal says hydroxychloroquine virus treatment is promising, https://medicalxpress.com/news/202..xychloroquine-virus-treatment.html.
195.Medical Xpress (B), Amid global controversy, Greece moves forward with chloroquine, https://medicalxpress.com/news/202..ontroversy-greece-chloroquine.html.
196.Membrillo de Novales et al., Preprints 2020, 2020050057, doi:10.20944/preprints202005.0057.v1, Early Hydroxychloroquine Is Associated with an Increase of Survival in COVID-19 Patients: An Observational Study, https://www.preprints.org/manuscript/202005.0057.
197.Meneguesso, A., Médica defende tratamento precoce da Covid-19, https://www.youtube.com/watch?v=X5FCrIm_19U.
198.Middle East Eye, Coronavirus: Turkey says hydroxychloroquine dramatically reduces pneumonia cases, https://www.middleeasteye.net/news..roquine-malaria-treatment-progress.
199.Mikami et al., J. Gen. Intern. Med., doi:10.1007/s11606-020-05983-z, Risk Factors for Mortality in Patients with COVID-19 in New York City, https://link.springer.com/article/10.1007/s11606-020-05983-z.
200.Ministerstva Zdravotnictví, Rozhodnutí o dočasném povolení neregistrovaného humánního léčivého přípravku HYDROXYCHLOROQUINE SULFATE TABLETS, https://www.mzcr.cz/rozhodnuti-o-d..ydroxychloroquine-sulfate-tablets/.
201.Ministry of Health of Ukraine, ПРОТОКОЛ «НАДАННЯ МЕДИЧНОЇ ДОПОМОГИ ДЛЯ ЛІКУВАННЯ КОРОНАВІРУСНОЇ ХВОРОБИ (COVID-19)» , https://www.dec.gov.ua/wp-content/..04/2020_762_protokol_covid19-f.pdf.
202.Ministry of Health of Ukraine (B), «НАДАННЯ МЕДИЧНОЇ ДОПОМОГИ ДЛЯ ЛІКУВАННЯ КОРОНАВІРУСНОЇ ХВОРОБИ (COVID-19), https://moz.gov.ua/uploads/5/26129-dn_2106_17_09_2020_dod_1.pdf.
203.Mitchell et al., SSRN, doi:10.2139/ssrn.3586954, Markedly Lower Rates of Coronavirus Infection and Fatality in Malaria-Endemic Regions – A Clue As to Treatment?, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3586954.
204.Mitjà et al., Clinical Infectious Diseases, ciaa1009, doi:10.1093/cid/ciaa1009, Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial, https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa1009/5872589.
205.Mitjà (B) et al., NEJM, doi:10.1056/NEJMoa2021801 (preprint 7/26), A Cluster-Randomized Trial of Hydroxychloroquine as Prevention of Covid-19 Transmission and Disease, https://www.nejm.org/doi/full/10.1056/NEJMoa2021801.
206.Modrák et al., medRxiv, doi:10.1101/2020.12.03.20239863, Detailed disease progression of 213 patients hospitalized with Covid-19 in the Czech Republic: An exploratory analysis, https://www.medrxiv.org/content/10.1101/2020.12.03.20239863v1.
207.Mokhtari et al., International Immunopharmacology, doi:10.1016/j.intimp.2021.107636, Clinical outcomes of patients with mild COVID-19 following treatment with hydroxychloroquine in an outpatient setting, https://www.sciencedirect.com/science/article/pii/S1567576921002721.
208.Morocco World News, Moroccan Scientist: Morocco’s Chloroquine Success Reveals European Failures, https://www.moroccoworldnews.com/2..success-reveals-european-failures/.
209.Mosaique Guinee, Traitement des malades de covid19 en Guinée: « nous continuons avec l’hydroxychloroquine » (ANSS), https://mosaiqueguinee.com/traitem..ons-avec-lhydroxychloroquine-anss/.
210.Nachega et al., The American Journal of Tropical Medicine and Hygiene, doi:10.4269/ajtmh.20-1240, Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 in Africa: Early Insights from the Democratic Republic of the Congo, https://www.ajtmh.org/content/journals/10.4269/ajtmh.20-1240.
211.Ñamendys-Silva et al., Heart & Lung, doi:10.1016/j.hrtlng.2020.10.013, Outcomes of patients with COVID-19 in the Intensive Care Unit in Mexico: A multicenter observational study, https://www.sciencedirect.com/science/article/pii/S014795632030412X.
212.Naseem et al., medRxiv, doi:10.1101/2020.12.13.20247254, Predicting mortality in SARS-COV-2 (COVID-19) positive patients in the inpatient setting using a Novel Deep Neural Network, https://www.medrxiv.org/content/10.1101/2020.12.13.20247254v1.
213.Nichol et al., Injury, 2010, doi: 10.1016/j.injury.2010.03.033, Challenging issues in randomised controlled trials, https://www.injuryjournal.com/article/S0020-1383(10)00233-0/fulltext.
214.Nigeria News World, COVID-19: Jigawa govt reveals secret behind mass recovery of patients, https://nigerianewsworld.com/news/..-behind-mass-recovery-of-patients/.
215.NPR News, Senegal pledges a bed for every coronavirus patient, https://wfuv.org/content/senegal-p..t-%E2%80%94-and-their-contacts-too.
216.Núñez-Gil et al., Intern. Emerg. Med., doi:10.1007/s11739-020-02543-5, Mortality risk assessment in Spain and Italy, insights of the HOPE COVID-19 registry, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7649104/.
217.Omrani et al., EClinicalMedicine, doi:10.1016/j.eclinm.2020.100645, Randomized double-blinded placebo-controlled trial of hydroxychloroquine with or without azithromycin for virologic cure of non-severe Covid-19, https://www.sciencedirect.com/science/article/pii/S2589537020303898.
218.Oneindia, No COVID-19 death in Manipur, Mizoram, Nagaland, Sikkim so far: Govt, https://www.oneindia.com/india/no-..o-far-health-ministry-3111048.html.
219.Orioli et al., Diabetes & Metabolic Syndrome: Clinical Research & Reviews, doi:10.1016/j.dsx.2020.12.020, Clinical characteristics and short-term prognosis of in-patients with diabetes and COVID-19: A retrospective study from an academic center in Belgium, https://www.sciencedirect.com/science/article/pii/S1871402120305154.
220.Ouedraogo et al., Revue des Maladies Respiratoires, doi:10.1016/j.rmr.2021.02.001, Factors associated with the occurrence of acute respiratory distress and death in patients with COVID-19 in Burkina Faso, https://www.sciencedirect.com/science/article/pii/S0761842521000383.
221.Ozturk et al., Nephrology Dialysis Transplantation, doi:10.1093/ndt/gfaa271, Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey, https://academic.oup.com/ndt/article/35/12/2083/6020341.
222.Paccoud et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaa791, Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital, https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa791/5859555.
223.Pan African Medical Journal, Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study, https://www.panafrican-med-journal.com/content/series/37/1/9/full/.
224.Parola et al., COVID-19 in Africa: What else?, https://www.mediterranee-infection..oads/2020/09/COVIDAfricaJOUMII.pdf.
225.Pasquini et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkaa321, Effectiveness of remdesivir in patients with COVID-19 under mechanical ventilation in an Italian ICU, https://academic.oup.com/jac/article/75/11/3359/5896161.
226.Peng et al., Nephrology Dialysis Transplantation, doi:10.1093/ndt/gfaa288, Early versus late acute kidney injury among patients with COVID-19—a multicenter study from Wuhan, China , https://academic.oup.com/ndt/article/35/12/2095/6020340.
227.Peters et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2020.10.004 (preprint 8/15), Outcomes of Persons With COVID-19 in Hospitals With and Without Standard Treatment With (Hydroxy)chloroquine, https://www.clinicalmicrobiologyan..cle/S1198-743X(20)30615-7/fulltext.
228.Pham et al., Rheumatology Advances in Practice, 10.1093/rap/rkab014, Failure of chronic hydroxychloroquine in preventing severe complications of COVID-19 in patients with rheumatic diseases, https://academic.oup.com/rheumap/a..le/doi/10.1093/rap/rkab014/6156645.
229.Pilot News, Chloroquine Can Treat Coronavirus at Early Stage – NAFDAC DG, https://www.westafricanpilotnews.c..onavirus-at-early-stage-nafdac-dg/.
230.Pinato et al., Cancer Discovery, doi:10.1158/2159-8290.CD-20-0773, Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients, https://cancerdiscovery.aacrjourna..ly/2020/08/18/2159-8290.CD-20-0773.
231.PledgeTimes, Russian Ministry of Health has updated recommendations for the treatment of COVID-19, https://pledgetimes.com/russian-mi..ons-for-the-treatment-of-covid-19/.
232.Pleno.News, Cuba stands out in combating Covid with hydroxychloroquine, https://pleno.news/saude/coronavir..a-covid-com-hidroxicloroquina.html.
233.Polat et al., Medical Journal of Bakirkoy, 16:3, 280-6, doi:10.5222/BMJ.2020.50469, Hydroxychloroquine Use on Healthcare Workers Exposed to COVID-19 -A Pandemic Hospital Experience, https://www.bakirkoytip.org/jvi.as..oytip&plng=eng&un=BMJ-50469&look4=.
234.Prodromos et al., New Microbes and New Infections, doi:10.1016/j.nmni.2020.100776, Hydroxychloroquine is effective, and consistently so used early, for Covid-19: A systematic review, https://www.sciencedirect.com/science/article/pii/S2052297520301281.
235.Psevdos et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa439.721, Corona Virus Disease-19 (COVID-19) in a Veterans Affairs Hospital at Suffolk County, Long Island, New York, https://academic.oup.com/ofid/article/7/Supplement_1/S330/6057008.
236.Purwati et al., Biochemistry Research International, doi:10.1155/2021/6685921, A Randomized, Double-Blind, Multicenter Clinical Study Comparing the Efficacy and Safety of a Drug Combination of Lopinavir/Ritonavir-Azithromycin, Lopinavir/Ritonavir-Doxycycline, and Azithromycin-Hydroxychloroquine for Patients Diagnosed with Mild to Moderate COVID-19 Infections, https://www.hindawi.com/journals/bri/2021/6685921/.
237.Q Costa Rica, Hydroxychloroquine: The Drug Costa Rica Uses Successfully To Fight Covid-19, https://qcostarica.com/hydroxychlo..es-successfully-to-fight-covid-19/.
238.Qin et al., Thrombosis Research, doi:10.1016/j.thromres.2020.11.020, Low molecular weight heparin and 28-day mortality among patients with coronavirus disease 2019: A cohort study in the early epidemic era, https://www.sciencedirect.com/science/article/pii/S0049384820306277.
239.Rajasingham et al., medRxiv, doi:10.1101/2020.09.18.20197327, Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, https://academic.oup.com/cid/advan..e/doi/10.1093/cid/ciaa1571/5929230.
240.Rangel et al., Journal of the American Academy of Dermatology, doi:10.1016/j.jaad.2020.10.098, Chronic Hydroxychloroquine Therapy and COVID-19 Outcomes: A Retrospective Case-Control Analysis, https://www.sciencedirect.com/science/article/pii/S0190962221001109.
241.Rathi et al. Lancet Infect. Dis. doi:10.1016/S1473-3099(20)30313-3, Hydroxychloroquine prophylaxis for COVID-19 contacts in India, https://www.thelancet.com/journals../PIIS1473-3099(20)30313-3/fulltext.
242.RECOVERY Collaborative Group, NEJM, doi:10.1056/NEJMoa2022926 (press release 6/5), Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial, https://www.nejm.org/doi/full/10.1056/NEJMoa2022926.
243.Rentsch et al., The Lancet Rheumatology, doi:10.1016/S2665-9913(20)30378-7 (preprint 9/9, https://www.medrxiv.org/content/10.1101/2020.09.04.20187781v1), Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform, https://www.sciencedirect.com/science/article/pii/S2665991320303787.
244.Revollo et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkaa477, Hydroxychloroquine pre-exposure prophylaxis for COVID-19 in healthcare workers, https://academic.oup.com/jac/advan..le/doi/10.1093/jac/dkaa477/5997449.
245.Rivera et al., Cancer Discovery, doi:10.1158/2159-8290.CD-20-0941, Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study, https://cancerdiscovery.aacrjourna..ly/2020/09/12/2159-8290.CD-20-0941.
246.Rivera-Izquierdo et al., Medicina Clínica, doi:10.1016/j.medcli.2020.06.025, Agentes terapéuticos utilizados en 238 pacientes hospitalizados por COVID-19 y su relación con la mortalidad, https://www.sciencedirect.com/science/article/pii/S0025775320304486.
247.Rodriguez et al., Medicina Intensiva, doi:10.1016/j.medine.2020.05.005, Severe infection due to the SARS-CoV-2 coronavirus: Experience of a tertiary hospital with COVID-19 patients during the 2020 pandemic, https://www.sciencedirect.com/science/article/pii/S2173572720301739.
248.Rodriguez-Gonzalez et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106249, COVID-19 in hospitalized patients in Spain: a cohort study in Madrid, https://www.sciencedirect.com/science/article/pii/S0924857920304696.
249.Rodriguez-Nava et al., Mayo Clinic Proceedings: Innovations, Quality & Outcomes, Clinical characteristics and risk factors for mortality of hospitalized patients with COVID-19 in a community hospital: A retrospective cohort study, https://www.sciencedirect.com/science/article/pii/S2542454820302071.
250.Roig et al., Revista Espanola de Quimioterapia, doi:10.37201/req/130.2020, Clinical and pharmacological data in COVID-19 hospitalized nonagenarian patients, https://europepmc.org/article/med/33522213.
251.Roomi et al., J. Medical Internet Research, doi:10.2196/21758, Efficacy of hydroxychloroquine and tocilizumab in patients with COVID-19: A single-center retrospective chart review, https://www.jmir.org/2020/9/e21758/.
252.Rosenberg et al., JAMA, May 11, 2020, doi:10.1001/jama.2020.8630, Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State, https://jamanetwork.com/journals/jama/fullarticle/2766117.
253.Roy et al., medRxiv, doi:10.1101/2021.03.08.21252883, Outcome of Different Therapeutic Interventions in Mild COVID-19 Patients in a Single OPD Clinic of West Bengal: A Retrospective study, https://www.medrxiv.org/content/10.1101/2021.03.08.21252883v1.
254.Russian Government, ВРЕМЕННЫЕ МЕТОДИЧЕСКИЕ РЕКОМЕНДАЦИИ ПРОФИЛАКТИКА, ДИАГНОСТИКА И ЛЕЧЕНИЕ НОВОЙ КОРОНАВИРУСНОЙ ИНФЕКЦИИ (COVID-19), https://static-0.minzdrav.gov.ru/s..D0%9C%D0%A0_COVID-19_%28v.9%29.pdf.
255.Russian Government (B), Распоряжение Правительства Российской Федерации от 16.04.2020 № 1030-р, http://publication.pravo.gov.ru/Document/View/0001202004160037#print.
256.Salazar et al., The American Journal of Pathology, doi:10.1016/j.ajpath.2020.10.008, Significantly Decreased Mortality in a Large Cohort of Coronavirus Disease 2019 (COVID-19) Patients Transfused Early with Convalescent Plasma Containing High-Titer Anti–Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein IgG, https://www.sciencedirect.com/science/article/pii/S0002944020304892.
257.Saleemi et al., medRxiv, doi:10.1101/2020.08.05.20151027, Time to negative PCR from symptom onset in COVID-19 patients on Hydroxychloroquine and Azithromycin – A real world experience, https://www.medrxiv.org/content/10.1101/2020.08.05.20151027v1.
258.Salvador et al., Cureus, doi:10.7759/cureus.13687, Clinical Features and Prognostic Factors of 245 Portuguese Patients Hospitalized With COVID-19, https://www.cureus.com/articles/53..atients-hospitalized-with-covid-19.
259.Salvarani et al., Arthritis & Rheumatology, doi:10.1002/art.41475, Susceptibility to COVID‐19 in Patients Treated With Antimalarials: A Population‐Based Study in Emilia‐Romagna, Northern Italy, https://onlinelibrary.wiley.com/doi/10.1002/art.41475.
260.Sánchez-Álvarez et al., Nefrología, doi:10.1016/j.nefroe.2020.04.002, Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN), https://www.sciencedirect.com/science/article/pii/S201325142030050X.
261.Sands et al., International Journal of Infectious Diseases, doi:/10.1016/j.ijid.2020.12.060, No clinical benefit in mortality associated with hydroxychloroquine treatment in patients with COVID-19, https://www.sciencedirect.com/science/article/pii/S1201971220325832.
262.Sarfaraz et al., medRxiv, doi:10.1101/2020.12.28.20248920, Determinants of in-hospital mortality in COVID-19; a prospective cohort study from Pakistan, https://www.medrxiv.org/content/10.1101/2020.12.28.20248920v1.
263.Sbidian et al., medRxiv, doi:10.1101/2020.06.16.20132597, Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France, https://www.medrxiv.org/content/10.1101/2020.06.16.20132597v1.
264.Seet et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2021.04.035, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial, https://www.ijidonline.com/article/S1201-9712(21)00345-3/fulltext.
265.Self et al., JAMA, doi:10.1001/jama.2020.22240, Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial, https://jamanetwork.com/journals/jama/fullarticle/2772922.
266.Serrano et al., Ann. Oncol., 2020, Sep, 31, S1026, doi:10.1016/j.annonc.2020.08.1830, COVID-19 and lung cancer: What do we know?, https://www.annalsofoncology.org/a..cle/S0923-7534(20)41826-5/fulltext.
267.Shabrawishi et al., medRxix, doi:10.1101/2020.05.08.20095679, Negative nasopharyngeal SARS-CoV-2 PCR conversion in response to different therapeutic interventions, https://www.medrxiv.org/content/10.1101/2020.05.08.20095679v1.
268.Sheshah et al., Diabetes Research and Clinical Practice, doi:10.1016/j.diabres.2020.108538, Prevalence of Diabetes, Management and Outcomes among Covid-19 Adult Patients Admitted in a Specialized Tertiary Hospital in Riyadh, Saudi Arabia, https://www.sciencedirect.com/science/article/pii/S0168822720307956.
269.Shoaibi et al., medRxiv, doi:10.1101/2020.09.23.20199463, Comparative Effectiveness of Famotidine in Hospitalized COVID-19 Patients, https://www.medrxiv.org/content/10.1101/2020.09.23.20199463v1.
270.Signes-Costa et al., Archivos de Bronconeumología, doi:10.1016/j.arbres.2020.11.012, Prevalence and 30-day mortality in hospitalized patients with COVID-19 and prior lung diseases, https://www.sciencedirect.com/science/article/pii/S0300289620305354.
271.Simova et al., New Microbes and New Infections, doi:10.1016/j.nmni.2020.100813, Hydroxychloroquine for prophylaxis and treatment of COVID-19 in health care workers, https://www.sciencedirect.com/science/article/pii/S2052297520301657.
272.Simova (B) et al., New Microbes and New Infections, doi:10.1016/j.nmni.2020.100813, Hydroxychloroquine for prophylaxis and treatment of COVID-19 in health care workers, https://www.sciencedirect.com/science/article/pii/S2052297520301657.
273.Singer et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-218500, Hydroxychloroquine ineffective for COVID-19 prophylaxis in lupus and rheumatoid arthritis, https://ard.bmj.com/content/early/2020/08/19/annrheumdis-2020-218500.
274.Singh et al., medRxiv, doi:10.1101/2020.05.12.20099028, Outcomes of Hydroxychloroquine Treatment Among Hospitalized COVID-19 Patients in the United States- Real-World Evidence From a Federated Electronic Medical Record Network, https://www.medrxiv.org/content/10.1101/2020.05.12.20099028v1.
275.Skipper et al., Annals of Internal Medicine, doi:10.7326/M20-4207, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial, https://www.acpjournals.org/doi/10.7326/M20-4207.
276.Solh et al., medRxiv, doi:10.1101/2020.10.16.20214130, Clinical course and outcome of COVID-19 acute respiratory distress syndrome: data from a national repository, https://www.medrxiv.org/content/10.1101/2020.10.16.20214130v1.
277.SOLIDARITY Trial Consortium, NEJM, doi:10.1056/NEJMoa2023184 (preprint 10/15), Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results, https://www.nejm.org/doi/full/10.1056/NEJMoa2023184.
278.Sosa-García et al., Cir Cir. 2020, 88:5, 569-575, doi:10.24875/CIRU.20000675, Experience in the management of severe COVID-19 patients in an intensive care unit, https://cirugiaycirujanos.com/frame_esp.php?id=358.
279.Soto-Becerra et al., medRxiv, doi:10.1101/2020.10.06.20208066, Real-World Effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: Results of a target trial emulation using observational data from a nationwide Healthcare System in Peru, https://www.medrxiv.org/content/10.1101/2020.10.06.20208066v1.
280.Stewart et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
281.Stewart (B) et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
282.Stewart (C) et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
283.Stewart (D) et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
284.Stewart (E) et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
285.Stewart (F) et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
286.Stewart (G) et al., PLoS ONE, doi:10.1371/journal.pone.0248128, COVID-19 Evidence Accelerator: A parallel analysis to describe the use of Hydroxychloroquine with or without Azithromycin among hospitalized COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0248128.
287.Su et al., BioScience Trends, doi:10.5582/bst.2020.03340, Efficacy of early hydroxychloroquine treatment in preventing COVID-19 pneumonia aggravation, the experience from Shanghai, China, https://www.jstage.jst.go.jp/artic..vpub_2020.03340/_article/-char/ja/.
288.Sulaiman et al., medRxiv, doi:10.1101/2020.09.09.20184143, The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study, https://www.medrxiv.org/content/10.1101/2020.09.09.20184143v1.
289.Sweeting et al., Statistics in Medicine, doi:10.1002/sim.1761, What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data, https://onlinelibrary.wiley.com/doi/10.1002/sim.1761.
290.Synolaki et al., medRxiv, doi:10.1101/2020.09.05.20184655, The Activin/Follistatin-axis is severely deregulated in COVID-19 and independently associated with in-hospital mortality, https://www.medrxiv.org/content/10.1101/2020.09.05.20184655v2.
291.Taccone et al., The Lancet Regional Health – Europe, doi:10.1016/j.lanepe.2020.100019, The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium, https://www.sciencedirect.com/science/article/pii/S2666776220300193.
292.Tan et al., Virus Research, doi:10.1016/j.virusres.2020.198262, A retrospective comparison of drugs against COVID-19, https://www.sciencedirect.com/scie../article/abs/pii/S0168170220311692.
293.Tang et al., BMJ 2020, 369, doi:10.1136/bmj.m1849, Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial, https://www.bmj.com/content/369/bmj.m1849.
294.Tehrani et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.10.071, Risk factors for mortality in adult COVID-19 patients; frailty predicts fatal outcome in older patients, https://www.sciencedirect.com/science/article/pii/S1201971220322761.
295.Teller Report, Coronavirus: a study in Senegal confirms the effectiveness of hydroxychloroquine, http://www.tellerreport.com/news/2..hydroxychloroquine.BJeet4Kst8.html.
296.Texeira et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa439.560, Characteristics and outcomes of COVID-19 patients admitted to a regional health system in the southeast, https://academic.oup.com/ofid/article/7/Supplement_1/S251/6058327.
297.The Africa Report, Coronavirus: Didier Raoult the African and chloroquine, from Dakar to Brazzaville, https://www.theafricareport.com/26..roquine-from-dakar-to-brazzaville/.
298.The Australian, India and Indonesia stand by antimalarials, https://www.theaustralian.com.au/w..y/d7856d1371697fe69e4fcc39d7f1f97c.
299.The BL, Russia supports the use of hydroxychloroquine, the drug to treat the CCP Virus suggested by Trump, https://thebl.com/world-news/russi..oroquine-drug-ccp-virus-trump.html.
300.The East African, Algeria backs use of malaria drug despite WHO dropping trials, https://www.theeastafrican.co.ke/n../4552902-5564930-duphp6/index.html.
301.The Guardian, Chloroquine potent for COVID-19 prevention, says NAFDAC, https://guardian.ng/news/nigeria/n..r-covid-19-prevention-says-nafdac/.
302.The Indian Express, Vadodara administration drive: HCQ helping in containing Covid-19 cases, say docs as analysis begins, https://indianexpress.com/article/..y-docs-as-analysis-begins-6486049/.
303.The Moscow Times, Russia Approves Unproven Malaria Drug to Treat Coronavirus, https://www.themoscowtimes.com/202..a-drug-to-treat-coronavirus-a70025.
304.The New York Times, Malaria Drug Taken by Trump Is Tied to Increased Risk of Heart Problems and Death in New Study, https://www.nytimes.com/2020/05/22..alaria-drug-trump-coronavirus.html.
305.The New York Times (B), Small Chloroquine Study Halted Over Risk of Fatal Heart Complications, https://www.nytimes.com/2020/04/12..ronavirus-trump.html?smid=em-share.
306.The New York Times (C), Malaria Drug Promoted by Trump Did Not Prevent Covid Infections, Study Finds, https://www.nytimes.com/2020/06/03..chloroquine-coronavirus-trump.html.
307.The New York Times (D), Coronavirus Can Be Deadly for Young Adults, Too, Study Finds, https://www.nytimes.com/2020/09/10/world/covid-19-coronavirus.html.
308.The North Africa Post, Morocco continues use of Chloroquine despite controversy, https://northafricapost.com/41247-..loroquine-despite-controversy.html.
309.The Tico Times, News briefs: Reopening plans on-track, hydroxychloroquine use to continue, partnership with Coursera, https://ticotimes.net/2020/06/15/n..continue-partnership-with-coursera.
310.Treanor et al., JAMA, 2000, 283:8, 1016-1024, doi:10.1001/jama.283.8.1016, Efficacy and Safety of the Oral Neuraminidase Inhibitor Oseltamivir in Treating Acute Influenza: A Randomized Controlled Trial, https://jamanetwork.com/journals/jama/fullarticle/192425.
311.Trefond et al., SSRM, doi:10.2139/ssrn.3754815, Impact of hydroxychloroquine used as DMARD on SARS CoV-2 tests and COVID-19 evolution, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3754815.
312.Trullàs et al., Research Square, doi:10.21203/rs.3.rs-39421/v1 , High in-hospital mortality due to COVID-19 in a community hospital in Spain: a prospective observational study, https://www.researchsquare.com/article/rs-39421/v1.
313.Ubaldo et al., Critical Care Research and Practice, 10.1155/2021/7510306, COVID-19: A Single-Center ICU Experience of the First Wave in the Philippines, https://www.hindawi.com/journals/ccrp/2021/7510306/.
314.Ukrinform, Ukraine receives batch of hydroxychloroquine tablets from India, https://www.ukrinform.net/rubric-e..ose-down-in-ukraine-on-june-3.html.
315.Ulrich et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa446, Treating Covid-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind, Randomized Controlled Trial in Hospitalized Patients, https://academic.oup.com/ofid/adva..e/doi/10.1093/ofid/ofaa446/5910201.
316.United States National Institutes of Health, Chloroquine or Hydroxychloroquine With or Without Azithromycin, https://www.covid19treatmentguidel..uine-with-or-without-azithromycin/.
317.van Halem et al., BMC Infect Dis., doi:10.1186/s12879-020-05605-3, Risk factors for mortality in hospitalized patients with COVID-19 at the start of the pandemic in Belgium: a retrospective cohort study, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7691970/.
318.Vanguard, COVID-19: Nigerian study finds Chloroquine, Hydroxychloroquine effective as Prophylaxis, https://www.vanguardngr.com/2020/0..oroquine-effective-as-prophylaxis/.
319.Vernaz et al., Swiss Medical Weekly, doi:10.4414/smw.2020.20446 , Early experimental COVID-19 therapies: associations with length of hospital stay, mortality and related costs, https://smw.ch/article/doi/smw.2020.20446.
320.Vivanco-Hidalgo et al., Eurosurveillance, doi:/10.2807/1560-7917.ES.2021.26.9.2001202, Incidence of COVID-19 in patients exposed to chloroquine and hydroxychloroquine: results from a population-based prospective cohort in Catalonia, Spain, 2020, https://www.eurosurveillance.org/c..807/1560-7917.ES.2021.26.9.2001202.
321.Voice of America, Cameroon Begins Large-scale Chloroquine Production, https://www.voanews.com/science-he..large-scale-chloroquine-production.
322.Wang et al., medRxiv, doi:10.1101/2020.06.11.20128926, Comorbidity and Sociodemographic determinants in COVID-19 Mortality in an US Urban Healthcare System, https://www.medrxiv.org/content/10.1101/2020.06.11.20128926v1.
323.Xia et al., ChiCTR2000029741, Efficacy of Chloroquine and Lopinavir/ Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study, http://www.chictr.org.cn/showproj.aspx?proj=49263.
324.Yegerov et al., medRxiv, doi:10.1101/2021.01.06.20249091, Epidemiological and Clinical Characteristics, and Virologic Features of COVID-19 Patients in Kazakhstan: a Nation-Wide, Retrospective, Cohort Study, https://www.medrxiv.org/content/10.1101/2021.01.06.20249091v1.
325.Yu et al., Science China Life Sciences, 2020 Aug 3, doi:10.1007/s11427-020-1782-1, Beneficial effects exerted by hydroxychloroquine in treating COVID-19 patients via protecting multiple organs, https://link.springer.com/article/10.1007/s11427-020-1782-1.
326.Yu (B) et al., Science China Life Sciences, 2020 Aug 3, doi:10.1007/s11427-020-1782-1, Beneficial effects exerted by hydroxychloroquine in treating COVID-19 patients via protecting multiple organs, https://link.springer.com/article/10.1007/s11427-020-1782-1.
327.Yu (C) et al., Science China Life Sciences, 2020 May 15, 1-7, doi:10.1007/s11427-020-1732-2, Low Dose of Hydroxychloroquine Reduces Fatality of Critically Ill Patients With COVID-19, https://link.springer.com/article/10.1007%2Fs11427-020-1732-2.
328.Zhang et al., JAMA, 80:19, 1690, doi:10.1001/jama.280.19.1690, What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes, https://jamanetwork.com/journals/jama/fullarticle/188182.
329.Zhong Nanshan (钟南山), Efficacy and safety of chloroquine for treatment of COVID-19. An open-label, multi-center, non-randomized trial, https://twitter.com/JamesTodaroMD/status/1243260720944480265.
330.Zhong (B) et al., Lancent Rheumatology, 10.1016/S2665-9913(20)30227-7, COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study, https://www.thelancet.com/journals../PIIS2665-9913(20)30227-7/fulltext.
Related: Houston doctor successfully treated over 20K patients with hydroxychloroquine
Act early with early Covid-19 treatment: Dr Vladimir Zelenko
The Chloroquine Wars Part X – A Discussion of the Insanity of the Chloroquine Wars

