Source: HCQ Meta
HCQ is effective for COVID-19. The probability that an ineffective treatment generated results as positive as the 115 studies to date is estimated to be 1 in 20 million (p = 0.000000049).
•Early treatment is most successful, with 100% of studies reporting a positive effect and an estimated reduction of 63% in the effect measured (death, hospitalization, etc.) using a random effects meta-analysis, RR 0.37 [0.30-0.47].
•100% of Randomized Controlled Trials (RCTs) for early, PrEP, or PEP treatment report positive effects, the probability of this happening for an ineffective treatment is 0.002.
•There is evidence of bias towards publishing negative results. Significantly more retrospective studies report negative results compared to prospective studies, p = 0.05.
•Significantly more studies in North America report negative results compared to the rest of the world, p = 0.005.
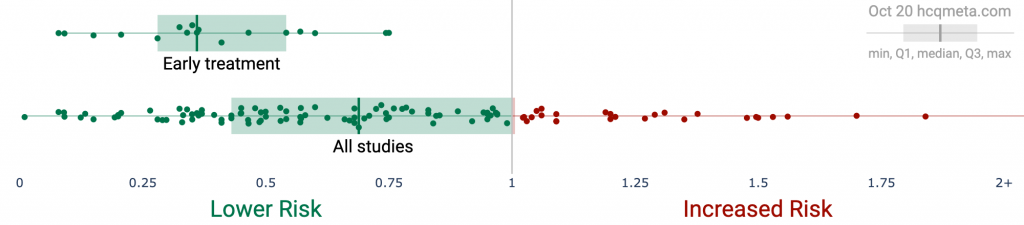


Figure 1. A. Scatter plot showing the distribution of effects reported in early treatment studies and in all studies. Early treatment is more effective. B and C. Study results ordered by date, with the line showing the probability that the observed frequency of positive results occurred due to random chance from an ineffective treatment.
Introduction
We analyze all significant studies concerning the use of HCQ (or CQ) for COVID-19 (Appendix 1), showing the effect size and associated p value for results comparing to a control group. Typical meta analyses involve subjective selection criteria and bias evaluation, requiring an understanding of the criteria and the accuracy of the evaluations. However, the volume of studies presents an opportunity for a simple and transparent analysis aimed at detecting efficacy.If treatment was not effective, the observed effects would be randomly distributed (or more likely to be negative if treatment is harmful). We can compute the probability that the observed percentage of positive results (or higher) could occur due to chance with an ineffective treatment (the probability of >= k heads in n coin tosses, or the one-sided sign test / binomial test). Analysis of publication bias is important and adjustments may be needed if there is a bias toward publishing positive results. For HCQ, we find evidence of a bias toward publishing negative results.

Figure 2 shows stages of possible treatment for COVID-19. Pre-Exposure Prophylaxis (PrEP) refers to regularly taking medication before being infected, in order to prevent or minimize infection. In Post-Exposure Prophylaxis (PEP), medication is taken after exposure but before symptoms appear. Early Treatment refers to treatment immediately or soon after symptoms appear, while Late Treatment refers to more delayed treatment.ResultsFigure 3, Figure 4 and Table 1 show results by treatment stage, and Figure 5 shows a forest plot for a random effects meta-analysis of all studies. Analysis excluding studies with major issues is in Appendix 2.Early treatment. 100% of early treatment studies report a positive effect, with an estimated reduction of 63% in the effect measured (death, hospitalization, etc.) from the random effects meta-analysis, RR 0.37 [0.30-0.47].Late treatment. Late treatment studies are mixed, with 68% showing positive effects, and an estimated reduction of 22% in the random effects meta-analysis. Negative studies mostly fall into the following categories: they show evidence of significant unadjusted confounding, including confounding by indication; usage is extremely late; or they use an excessively high dosage.Pre-Exposure Prophylaxis. 74% of PrEP studies are positive, with an estimated reduction of 41% in the random effects meta-analysis. Negative studies are all studies of systemic autoimmune disease patients which either do not adjust for the different baseline risk of these patients at all, or do not adjust for the highly variable risk within these patients.Post-Exposure Prophylaxis. 100% of PEP studies are positive, with an estimated reduction of 31% in the random effects meta-analysis.
| Treatment time | Number of positive studies | Total number of studies | Percentage of positive studies | Probability of an equal or greater percentage of positive results due to random chance | Random effects meta-analysis results |
| Early treatment | 19 | 19 | 100% | 0.0000019 1 in 524 thousand | 63% improvement RR 0.37 [0.30‑0.47] |
| Late treatment | 49 | 72 | 68.1% | 0.0015 1 in 680 | 22% improvement RR 0.78 [0.70‑0.87] |
| Pre‑Exposure Prophylaxis | 17 | 23 | 73.9% | 0.017 1 in 58 | 41% improvement RR 0.59 [0.43‑0.81] |
| Post‑Exposure Prophylaxis | 3 | 3 | 100% | 0.13 1 in 8 | 31% improvement RR 0.69 [0.46‑1.03] |
| All studies | 86 | 115 | 74.8% | 0.000000049 1 in 20 million | 32% improvement RR 0.68 [0.62‑0.75] |
Table 1. Results by treatment stage. 2 studies report results for a subset with early treatment, these are not included in the overall results.

Figure 3. Results by treatment stage.




Figure 4. Results by treatment stage. Study results are ordered by date, with the line showing the probability that the observed frequency of positive results occurred due to random chance from an ineffective treatment.
Figure 5. Forest plot (random effects model). (ES) indicates the early treatment subset of a study (these are not included in the overall results).Randomized Controlled Trials (RCTs)RCTs are very valuable and minimize potential bias, however they are neither necessary or sufficient. [Concato] find that well-designed observational studies do not systematically overestimate the magnitude of the effects of treatment compared to RCTs. [Lee] shows that only 14% of the guidelines of the Infectious Diseases Society of America were based on RCTs. Limitations in an RCT can easily outweigh the benefits, for example excessive dosages, excessive treatment delays, or Internet survey bias could easily have a greater effect on results. Ethical issues may prevent running RCTs for known effective treatments. For more on the problems with RCTs see [Deaton, Nichol]. Results restricted to RCTs are shown in Figure 6. Even with the small number of RCTs to date, there is a strong indication of efficacy. When excluding late treatment, 100% of RCTs to date report positive results.


Figure 6. Randomized Controlled Trials. The distribution of results for RCTs is similar to the distribution for all other studies.


Figure 7. RCTs excluding late treatment.
Publication bias. Publishing is often biased towards positive results, which we would need to adjust for when analyzing the percentage of positive results. Studies that require less effort are considered to be more susceptible to publication bias. Prospective trials that involve significant effort are likely to be published regardless of the result, while retrospective studies are more likely to exhibit bias. For example, researchers may perform preliminary analysis with minimal effort and the results may influence their decision to continue. Retrospective studies also provide more opportunities for the specifics of data extraction and adjustments to influence results.For HCQ, 87.1% of prospective studies report positive effects, compared to 70.2% of retrospective studies, two-tailed z test 1.96, p = 0.05, indicating a bias toward publishing negative results. Figure 8 shows a scatter plot of results for prospective and retrospective studies.Figure 9 shows the results by region of the world, for all regions that have > 5 studies. Studies from North America are significantly more likely to report negative results than studies from the rest of the world combined, two-tailed z test -2.79, p = 0.005. [Berry] perform an independent analysis which also shows a bias toward negative results for US-based research.

Figure 8. Prospective vs. retrospective studies.

Figure 9. Results by region.
The lack of bias towards positive results is not very surprising. Both negative and positive results are very important given the current use of HCQ for COVID-19 around the world, evidence of which can be found in the studies analyzed here, government protocols, and news reports, for example [AFP, AfricaFeeds, Africanews, Afrik.com, Al Arabia, Al-bab, Anadolu Agency, Anadolu Agency (B), Archyde, Barron’s, Barron’s (B), BBC, Belayneh, A., CBS News, Challenge, Dr. Goldin, Efecto Cocuyo, Expats.cz, Face 2 Face Africa, France 24, France 24 (B), Franceinfo, Global Times, Government of China, Government of India, GulfInsider, Le Nouvel Afrik, LifeSiteNews, Medical World Nigeria, Medical Xpress, Medical Xpress (B), Middle East Eye, Ministerstva Zdravotnictví, Morocco World News, Mosaique Guinee, Nigeria News World, NPR News, Oneindia, Pan African Medical Journal, Parola, Pilot News, Pleno.News, Q Costa Rica, Rathi, Russian Government, Teller Report, The Africa Report, The Australian, The BL, The East African, The Guardian, The Indian Express, The Moscow Times, The North Africa Post, The Tico Times, Ukraine Ministry of Health Care, Ukrinform, Vanguard, Voice of America].
We also note a bias towards publishing negative results by certain journals and press organizations, with scientists reporting difficulty publishing positive results [Boulware, Meneguesso]. Although 86 studies show positive results, The New York Times, for example, has only written articles for studies that claim HCQ is not effective [The New York Times, The New York Times (B), The New York Times (C)]. As of September 10, 2020, The New York Times still claims that there is clear evidence that HCQ is not effective for COVID-19 [The New York Times (D)].
Treatment details. We focus here on the question of whether HCQ is effective or not for COVID-19. Significant differences exist based on treatment stage, with early treatment showing the greatest effectiveness. 100% of early treatment studies report a positive effect, with an estimated reduction of 63% in the effect measured (death, hospitalization, etc.) in the random effects meta-analysis, RR 0.37 [0.30-0.47]. Many factors are likely to influence the degree of effectiveness, including the dosing regimen, concomitant medications such as zinc or azithromycin, precise treatment delay, the initial viral load of patients, and current patient conditions.
Conclusion
HCQ is an effective treatment for COVID-19. The probability that an ineffective treatment generated results as positive as the 115 studies to date is estimated to be 1 in 20 million (p = 0.000000049).
Revisions
This paper is data driven, all graphs and numbers are dynamically generated. We will update the paper as new studies are released or with any corrections.
References
1.Abd-Elsalam et al., American Journal of Tropical Medicine and Hygiene, 10.4269/ajtmh.20-0873, Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study, https://www.ajtmh.org/content/journals/10.4269/ajtmh.20-0873.
2.Abella et al., JAMA Internal Medicine, doi:doi:10.1001/jamainternmed.2020.6319, Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers, https://jamanetwork.com/journals/j..ternalmedicine/fullarticle/2771265.
3.AFP, India backs hydroxychloroquine for virus prevention, https://www.msn.com/en-ph/news/wor..us-prevention/ar-BB14EloP?ocid=st2.
4.AfricaFeeds, Kenya approve the use of Chloroquine to treat COVID-19 patients, https://africafeeds.com/2020/04/01..oquine-to-treat-covid-19-patients/.
5.Africanews, Coronavirus patients on chloroquine heal faster – Senegalese medic, https://www.africanews.com/2020/04..uine-heal-faster-senegalese-medic/.
6.Afrik.com, Edouard Philippe emporté par le Covid, Didier Raoult, l’hydroxychloroquine et le… remdésivir, https://www.afrik.com/edouard-phil..ydroxychloroquine-et-le-remdesivir.
7.Al Arabia, Bahrain among first countries to use Hydroxychloroquine to treat coronavirus, https://english.alarabiya.net/en/N..xychloroquine-to-treat-coronavirus.
8.Al-bab, Covid-19: Algeria and Morocco continue using chloroquine despite concerns, https://al-bab.com/blog/2020/05/co..using-chloroquine-despite-concerns.
9.Alamdari et al., Tohoku J. Exp. Med., 2020, 252, 73-84, doi:10.1620/tjem.252.73, Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran, https://www.jstage.jst.go.jp/artic..em/252/1/252_73/_article/-char/ja/.
10.Alberici et al., Kidney Int., 98:1, 20-26, July 1, 2020, doi:10.1016/j.kint.2020.04.030 (preprint 5/10), A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection, https://www.kidney-international.o..cle/S0085-2538(20)30508-1/fulltext.
11.Almazrou et al., Saudi Pharmaceutical Journal, doi:10.1016/j.jsps.2020.09.019, Comparing the impact of Hydroxychloroquine based regimens and standard treatment on COVID-19 patient outcomes: A retrospective cohort study, https://www.sciencedirect.com/science/article/pii/S1319016420302334.
12.Altman, D., BMJ, doi:10.1136/bmj.d2304, How to obtain the P value from a confidence interval, https://www.bmj.com/content/343/bmj.d2304.
13.An et al., medRxiv, doi:10.1101/2020.07.04.20146548, Treatment Response to Hydroxychloroquine and Antibiotics for mild to moderate COVID-19: a retrospective cohort study from South Korea, https://www.medrxiv.org/content/10.1101/2020.07.04.20146548v1.
14.Anadolu Agency, Nigeria goes on with hydroxychloroquine clinical trial, https://www.aa.com.tr/en/africa/ni..hloroquine-clinical-trials/1854814.
15.Anadolu Agency (B), Cuba: Early hydroxychloroquine potent against COVID-19, https://www.aa.com.tr/en/americas/..ne-potent-against-covid-19/1905650.
16.Annie et al., Pharmacotherapy, doi:10.1002/phar.2467, Hydroxychloroquine in hospitalized COVID‐19 patients: Real world experience assessing mortality, https://accpjournals.onlinelibrary.wiley.com/doi/10.1002/phar.2467.
17.Aparisi et al., medRxiv, doi:10.1101/2020.10.06.20207092, Low-density lipoprotein cholesterol levels are associated with poor clinical outcomes in COVID-19, https://www.medrxiv.org/content/10.1101/2020.10.06.20207092v1.
18.Archyde, China approves chloroquine (instead of hydroxychloroquine) against covid-19, https://www.archyde.com/china-appr..droxychloroquine-against-covid-19/.
19.Arshad et al., Int. J. Infect. Dis., July 1 2020, doi:10.1016/j.ijid.2020.06.099, Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19, https://www.ijidonline.com/article/S1201-9712(20)30534-8/fulltext.
20.Ashinyo et al., Pan African Medical Journal, 37:1, doi:10.11604/pamj.supp.2020.37.1.25718, Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study, https://www.panafrican-med-journal.com/content/series/37/1/9/full/.
21.Ashraf et al., medRxiv doi:10.1101/2020.04.20.20072421.t, COVID-19 in Iran, a comprehensive investigation from exposure to treatment outcomes, https://www.researchgate.net/publi..rom_exposure_to_treatment_outcomes.
22.Ayerbe et al., Internal and Emergency Medicine, doi:0.1007/s11739-020-02505-x, The association of treatment with hydroxychloroquine and hospital mortality in COVID-19 patients, https://link.springer.com/article/10.1007/s11739-020-02505-x.
23.Barbosa et al., Preprint, Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study, https://www.sefq.es/_pdfs/NEJM_Hydroxychlorquine.pdf.
24.Barron’s, Hydroxychloroquine: A Drug Dividing The World, https://www.barrons.com/news/hydro..rug-dividing-the-world-01591006809.
25.Barron’s (B), Amid Global Controversy, Greece Moves Forward With Chloroquine, https://www.barrons.com/news/amid-..rward-with-chloroquine-01591781707.
26.BBC, Coronavirus: How Turkey took control of Covid-19 emergency, https://www.bbc.com/news/world-europe-52831017.
27.Belayneh, A., Off-Label Use of Chloroquine and Hydroxychloroquine for COVID-19 Treatment in Africa Against WHO Recommendation, https://www.dovepress.com/off-labe..eer-reviewed-fulltext-article-RRTM.
28.Bernaola et al., medRxiv, doi:10.1101/2020.07.17.20155960, Observational Study of the Efficiency of Treatments in Patients Hospitalized with Covid-19 in Madrid, https://www.medrxiv.org/content/10.1101/2020.07.17.20155960v1.
29.Berry et al., SSRN, Berry, doi:10.2139/ssrn.3707327., Unfavorable Hydroxychloroquine COVID-19 Research Associated with Authors Having a History of Political Party Donations, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3707327.
30.Bhattacharya et al., medRxix, doi:10.1101/2020.06.09.20116806, Pre exposure Hydroxychloroquine use is associated with reduced COVID19 risk in healthcare workers, https://www.medrxiv.org/content/10.1101/2020.06.09.20116806v1.
31.Boulware, D., Comments regarding paper rejection, https://twitter.com/boulware_dr/status/1311331372884205570.
32.Boulware (B) et al., NEJM, June 3 2020, doi:10.1056/NEJMoa2016638, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, https://www.nejm.org/doi/full/10.1056/NEJMoa2016638.
33.Bousquet et al., Aging, 12:12, 11306-11313, doi:10.18632/aging.103583, ADL-dependency, D-Dimers, LDH and absence of anticoagulation are independently associated with one-month mortality in older inpatients with Covid-19, https://www.aging-us.com/article/103583/text.
34.Cassione et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217717, COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine, https://ard.bmj.com/content/early/..05/23/annrheumdis-2020-217717.info.
35.Catteau et al., Int. J. Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106144, Low-dose Hydroxychloroquine Therapy and Mortality in Hospitalized Patients with COVID-19: A Nationwide Observational Study of 8075 Participants, https://www.sciencedirect.com/scie../article/abs/pii/S0924857920303423.
36.Cavalcanti et al., NEJM, July 23, 2020, doi:10.1056/NEJMoa201901, Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, https://www.nejm.org/doi/full/10.1056/NEJMoa2019014.
37.CBS News, Turkey claims success treating virus with drug touted by Trump, https://www.msn.com/en-au/news/wor..h-drug-touted-by-trump/ar-BB13oMXS.
38.Challenge, Coronavirus : ce que le Maroc a réussi, https://www.challenge.ma/coronavirus-ce-que-le-maroc-a-reussi-144484/.
39.Chatterjee et al., Indian J. Med. Res., June 20, 2020, doi:10.4103/ijmr.IJMR_2234_20, Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19, http://www.ijmr.org.in/preprintarticle.asp?id=285520.
40.Chen et al., medRxiv, doi:10.1101/2020.06.19.20136093, Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study, https://www.medrxiv.org/content/10.1101/2020.06.19.20136093v1.
41.Chen (B) et al., medRxiv, doi:10.1101/2020.07.08.20148841v1, A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19), https://www.medrxiv.org/content/10.1101/2020.07.08.20148841v1.
42.Chen (C) et al., medRxiv, doi:10.1101/2020.07.08.20148841v1, A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19), .
43.Chen (D) et al., medRxiv doi:10.1101/2020.03.22.20040758, Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3.
44.Chen (E) et al., J. Zhejiang University (Med Sci), doi:10.3785/j.issn.1008-9292.2020.03.03, A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19), http://www.zjujournals.com/med/EN/..cleFile.do?attachType=PDF&id=41137.
45.Concato et al., NEJM, 342:1887-1892, doi:10.1056/NEJM200006223422507, https://www.nejm.org/doi/full/10.1056/nejm200006223422507.
46.Cravedi et al., American Journal of Transplantation, doi:10.1111/ajt.16185, COVID‐19 and kidney transplantation: Results from the TANGO International Transplant Consortium, https://onlinelibrary.wiley.com/doi/full/10.1111/ajt.16185.
47.D’Arminio Monforte et al., Int. J. Infectious Diseases, doi:10.1016/j.ijid.2020.07.056, Effectiveness of Hydroxychloroquine in COVID-19 disease: A done and dusted situation?, https://www.ijidonline.com/article/S1201-9712(20)30600-7/fulltext.
48.Davido et al., Int. J. Antimicrobial Agents, 2020, doi:10.1016/j.ijantimicag.2020.106129, Impact of medical care including anti-infective agents use on the prognosis of COVID-19 hospitalized patients over time, https://www.sciencedirect.com/science/article/pii/S0924857920303125.
49.de la Iglesia et al., medRxiv, doi:10.1101/2020.08.31.20185314, Hydroxicloroquine for pre-exposure prophyylaxis for SARS-CoV-2, https://www.medrxiv.org/content/10.1101/2020.08.31.20185314v1.
50.Deaton et al., Social Science & Medicine, 210, doi:10.1016/j.socscimed.2017.12.005, Understanding and misunderstanding randomized controlled trials, https://www.sciencedirect.com/science/article/pii/S0277953617307359.
51.Deng, H., PyMeta, Python module for meta-analysis, http://www.pymeta.com/.
52.Di Castelnuovo et al., European J. Internal Medicine, doi:10.1016/j.ejim.2020.08.019, Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study, https://www.sciencedirect.com/scie../article/abs/pii/S0953620520303356.
53.DISCOVERY Trial, DISCOVERY Trial Preliminary Results, https://twitter.com/raoult_didier/status/1313509242167529472.
54.Dr. Goldin, Summary of HCQ usage in India from an MD in India, https://www.facebook.com/groups/hy..oquine/permalink/2367454293560817/.
55.Dubernet et al., J. Global Antimicrobial Resistance, doi:10.1016/j.jgar.2020.08.001, A comprehensive strategy for the early treatment of COVID-19 with azithromycin/hydroxychloroquine and/or corticosteroids: results of a retrospective observational study in the French overseas department of Reunion Island, https://www.sciencedirect.com/science/article/pii/S221371652030206X.
56.Efecto Cocuyo, Venezuela empieza a usar la cloroquina para tratar COVID-19, anuncia Jorge Rodríguez, https://efectococuyo.com/coronavir..-covid-19-anuncia-jorge-rodriguez/.
57.Esper et al., Prevent Senior Institute, São Paulo, Brazil, Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine, https://www.dropbox.com/s/5qm58cd4..20journal%20manuscript%20final.pdf.
58.Expats.cz, Czech Health Ministry permits temporary use of hydroxychloroquine to treat COVID-19, https://news.expats.cz/weekly-czec..ne-in-hospitals-to-treat-covid-19/.
59.Face 2 Face Africa, Djibouti, others warned about chloroquine despite big COVID-19 recoveries, https://face2faceafrica.com/articl..ne-despite-big-covid-19-recoveries.
60.Ferreira et al., J. Medical Virology, July 9, 2020, doi:10.1002/jmv.26286 (preprint 6/29), Chronic treatment with hydroxychloroquine and SARS-CoV-2 infection, https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.26286.
61.Ferri at al., Clinical Rheumatology, doi:0.1007/s10067-020-05334-7, COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series, https://link.springer.com/article/10.1007/s10067-020-05334-7.
62.Fontana et al., Clinical Kidney Journal, 13:3, 334–339, doi:10.1093/ckj/sfaa084, SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience, https://academic.oup.com/ckj/article/13/3/334/5860798.
63.France 24, Covid-19: In Cameroon, chloroquine therapy hailed by French expert becomes state protocol, https://www.france24.com/en/202005..ench-expert-becomes-state-protocol.
64.France 24 (B), Covid-19 : au Cameroun, la méthode Raoult érigée en protocole d’État, https://www.france24.com/fr/202005..ig%C3%A9e-en-protocole-d-%C3%A9tat.
65.Franceinfo, Ces pays africains qui ont décidé de continuer à soigner le Covid-19 avec l’hydroxychloroquine, https://www.francetvinfo.fr/monde/..-l-hydroxychloroquine_3983239.html.
66.Fried et al., Clinical Infectious Disease, doi:10.1093/cid/ciaa1268, Patient Characteristics and Outcomes of 11,721 Patients with COVID19 Hospitalized Across the United States, https://academic.oup.com/cid/advan..e/doi/10.1093/cid/ciaa1268/5898276.
67.Gautret et al., Int. J. of Antimicrobial Agents, 17 March 2020, doi:10.1016/j.ijantimicag.2020.105949, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial, https://www.mediterranee-infection..roxychloroquine_final_DOI_IJAA.pdf.
68.Geleris et al., NEJM, May 7, 2020, doi:10.1056/NEJMoa2012410, Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19, https://www.nejm.org/doi/full/10.1056/NEJMoa2012410.
69.Gendebien et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-218244, Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments, https://ard.bmj.com/content/early/2020/06/25/annrheumdis-2020-218244.
70.Gendelman et al., Autoimmunity Reviews, 19:7, July 2020, doi:10.1016/j.autrev.2020.102566, Continuous Hydroxychloroquine or Colchicine Therapy Does Not Prevent Infection With SARS-CoV-2: Insights From a Large Healthcare Database Analysis, https://www.sciencedirect.com/science/article/pii/S1568997220301282.
71.Gentry et al., Lancet Rheumatology, doi:10.1016/S2665-9913(20)30305-2, Long-term hydroxychloroquine use in patients with rheumatic conditions and development of SARS-CoV-2 infection: a retrospective cohort study, https://www.thelancet.com/journals../PIIS2665-9913(20)30305-2/fulltext.
72.Gianfrancesco et al., Annals of the Rheumatic Diseases, 79:7, 859-866, doi:10.1136/annrheumdis-2020-217871, Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry, https://europepmc.org/article/med/32471903.
73.Global Times, Chinese medical expert decorated by Djibouti for COVID-19 prevention, https://www.globaltimes.cn/content/1189839.shtml.
74.Goldman et al., NEJM, doi:10.1056/NEJMoa2015301, Remdesivir for 5 or 10 Days in Patients with Severe Covid-19, https://www.nejm.org/doi/10.1056/NEJMoa2015301.
75.Gonzalez et al., medRxiv, doi:10.1101/2020.08.18.20172874, The Prognostic Value of Eosinophil Recovery in COVID-19: A Multicentre, Retrospective Cohort Study on Patients Hospitalised in Spanish Hospitals, https://www.medrxiv.org/content/10.1101/2020.08.18.20172874v1.
76.Government of China, 关于印发新型冠状病毒肺炎诊疗方案(试行第八版)的通知, http://www.nhc.gov.cn/yzygj/s7653p..df12bd4b46e5bd28ca7f9a7f5e5a.shtml.
77.Government of India, The caregiver and all close contacts of such cases should take HCQ prophylaxis, https://www.mohfw.gov.in/pdf/RevisedHomeIsolationGuidelines.pdf.
78.Grau-Pujol et al., Research Square, doi:10.21203/rs.3.rs-72132/v1, Pre-exposure prophylaxis with hydroxychloroquine for COVID-19: initial results of a double-blind, placebo-controlled randomized clinical trial, https://www.researchsquare.com/article/rs-72132/v1.
79.Guisado-Vasco, Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: A retrospective observational study (COQUIMA cohort), https://www.sciencedirect.com/science/article/pii/S2589537020303357.
80.Guisado-Vasco (B), Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: A retrospective observational study (COQUIMA cohort), https://www.sciencedirect.com/science/article/pii/S2589537020303357.
81.GulfInsider, Coronavirus: Bahrain’s Therapeutic Medication Proved Effective, https://www.gulf-insider.com/coron..eutic-medication-proved-effective/.
82.Gupta et al., JAMA Intern. Med., doi:10.1001/jamainternmed.2020.3596, Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US, https://jamanetwork.com/journals/j..ternalmedicine/fullarticle/2768602.
83.Guérin et al., Asian J. Medicine and Health, July 15, 2020, doi:10.9734/ajmah/2020/v18i730224 (preprint 5/31), Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19, https://www.journalajmah.com/index.php/AJMAH/article/view/30224.
84.Heberto et al., IJC Heart & Vasculature, doi:10.1016/j.ijcha.2020.100638, Implications of myocardial injury in Mexican hospitalized patients with coronavirus disease 2019 (COVID-19), https://www.sciencedirect.com/science/article/pii/S2352906720303365.
85.Heras et al., Research Square, doi:10.21203/rs.3.rs-70219/v1, COVID-19 mortality risk factors in older people in a long-term care center, https://www.researchsquare.com/article/rs-70219/v1.
86.Hong et al., Infect. Chemother., 2020, doi:10.3947/ic.2020.52.e43, Early Hydroxychloroquine Administration for Rapid Severe Acute Respiratory Syndrome Coronavirus 2 Eradication, https://icjournal.org/DOIx.php?id=10.3947/ic.2020.52.3.396.
87.Huang et al., Annals of the Rheumatic Diseases 2020:79, 1163-1169, doi:10.1136/annrheumdis-2020-217425, Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study, https://ard.bmj.com/content/79/9/1163.
88.Huang (B) et al., National Science Review, nwaa113, doi:10.1093/nsr/nwaa113, Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19, https://academic.oup.com/nsr/advan..le/doi/10.1093/nsr/nwaa113/5848167.
89.Huang (C) et al., Journal of Molecular Cell Biology, Volume 12, Issue 4, April 2020, 322–325, doi:10.1093/jmcb/mjaa014, Treating COVID-19 with Chloroquine, https://academic.oup.com/jmcb/article/12/4/322/5814655.
90.Huang (D) et al., National Science Review, nwaa113, doi:10.1093/nsr/nwaa113, Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19, https://academic.oup.com/nsr/advan..le/doi/10.1093/nsr/nwaa113/5848167.
91.Huh et al., medRxiv, doi:10.1101/2020.05.04.20089904, Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea, https://www.medrxiv.org/content/10.1101/2020.05.04.20089904v2.
92.Ip et al., medRxiv, doi:10.1101/2020.08.20.20178772, Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study, https://www.medrxiv.org/content/10.1101/2020.08.20.20178772v1.
93.Ip (B) et al., medRxiv, doi:10.1101/2020.05.21.20109207, Hydroxychloroquine and Tocilizumab Therapy in COVID-19 Patients – An Observational Study, https://www.medrxiv.org/content/10.1101/2020.05.21.20109207v1.
94.Izoulet M., SSRN, doi:10.2139/ssrn.3575899, Countries which Primarily Use Antimalarial Drugs As COVID-19 Treatment See Slower Dynamic of Daily Deaths, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3575899.
95.Kamran et al., medRxiv, doi:10.1101/2020.07.30.20165365, Clearing the fog: Is HCQ effective in reducing COVID-19 progression: A randomized controlled trial, https://www.medrxiv.org/content/10.1101/2020.07.30.20165365v1.
96.Kelly et al., British Journal of Clinical Pharmacology, doi:10.1111/bcp.14482, Clinical outcomes and adverse events in patients hospitalised with COVID‐19, treated with off‐label hydroxychloroquine and azithromycin, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bcp.14482.
97.Khurana et al., medRxiv, doi:10.1101/2020.07.21.20159301, Prevalence and clinical correlates of COVID-19 outbreak among healthcare workers in a tertiary level hospital, https://www.medrxiv.org/content/10.1101/2020.07.21.20159301v1.
98.Kim et al., medRxiv, doi:10.1101/2020.05.13.20094193, Treatment Response to Hydroxychloroquine, Lopinavir/Ritonavir, and Antibiotics for Moderate COVID 19: A First Report on the Pharmacological Outcomes from South Korea, https://www.medrxiv.org/content/10..20.05.13.20094193v1?versioned=true.
99.Kirenga et al., BMJ Open Respiratory Research, doi:10.1136/bmjresp-2020-000646, Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda, https://bmjopenrespres.bmj.com/content/7/1/e000646.
100.Konig et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217690, Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19, https://ard.bmj.com/content/early/2020/05/20/annrheumdis-2020-217690.
101.Kuderer et al., Lancet, June 20, 2020, doi:10.1016/S0140-6736(20)31187-9 (preprint 5/28), Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study, https://www.thelancet.com/journals../PIIS0140-6736(20)31187-9/fulltext.
102.Lagier et al., Travel Med. Infect. Dis. 101791, Jun 25, 2020, doi:10.1016/j.tmaid.2020.101791, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis, https://www.sciencedirect.com/science/article/pii/S1477893920302817.
103.Lammers et al., Int. J. Infectious Diseases, doi:10.1016/j.ijid.2020.09.1460, https://www.sciencedirect.com/science/article/pii/S1201971220321755.
104.Laplana et al., medRxiv, doi:10.1101/2020.09.03.20158121, Lack of protective effect of chloroquine derivatives on COVID-19 disease in a Spanish sample of chronically treated patients, https://www.medrxiv.org/content/10.1101/2020.09.03.20158121v1.
105.Lauriola et al., Clinical and Translational Science, doi:10.1111/cts.12860, Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in COVID‐19 patients, https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1111/cts.12860.
106.Le Nouvel Afrik, Covid-19 : pourquoi les Marocains décèdent plus en Europe qu’au Maroc, https://www.afrik.com/covid-19-pou..ecedent-plus-en-europe-qu-au-maroc.
107.Lecronier et al., Critical Care, 24:418, 2020, doi:10.1186/s13054-020-03117-9, Comparison of hydroxychloroquine, lopinavir/ritonavir, and standard of care in critically ill patients with SARS-CoV-2 pneumonia: an opportunistic retrospective analysis, https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03117-9.
108.Lee et al., Arch Intern Med., 2011, 171:1, 18-22, doi:10.1001/archinternmed.2010.482, Analysis of Overall Level of Evidence Behind Infectious Diseases Society of America Practice Guidelines, https://jamanetwork.com/journals/j..nternalmedicine/fullarticle/226373.
109.LifeSiteNews, Doctors insist this cheap, safe drug is “key to preventing huge loss of life” from Wuhan virus, https://www.lifesitenews.com/news/..huge-loss-of-life-from-covid-virus.
110.Luo et al., Annals of Oncology, 31:10, 1386-1396, doi:10.1016/j.annonc.2020.06.007, COVID-19 in patients with lung cancer, https://www.annalsofoncology.org/a..cle/S0923-7534(20)39894-X/fulltext.
111.Ly et al., Preprint, 2020, Pattern of SARS-CoV-2 infection among dependant elderly residents living in retirement homes in Marseille, France, March-June 2020, https://www.mediterranee-infection..D-Covid-19-Marseille-v20200821.pdf.
112.Lyngbakken et al., Research Square, doi:10.21203/rs.3.rs-44055/v1, https://www.researchsquare.com/article/rs-44055/v1.
113.Macias et al., medRxiv, 10.1101/2020.05.16.20104141, Similar incidence of Coronavirus Disease 2019 (COVID-19) in patients with rheumatic diseases with and without hydroxychloroquine therapy, https://www.medrxiv.org/content/10.1101/2020.05.16.20104141v1.
114.Magagnoli et al., Med (2020), doi:10.1016/j.medj.2020.06.001 (preprint 4/21), Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, https://www.sciencedirect.com/science/article/pii/S2666634020300064.
115.Mahévas et al., BMJ 2020, 369, doi: https://doi.org/10.1136/bmj.m1844, Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data, https://www.bmj.com/content/369/bmj.m1844.
116.McGrail et al., medRxiv, doi:10.1101/2020.07.17.20156521, COVID-19 Case Series at UnityPoint Health St. Luke’s Hospital in Cedar Rapids, IA, https://www.medrxiv.org/content/10.1101/2020.07.17.20156521v1.
117.McLean et al., Open Forum Infect. Dis. September 2015, 2:3, doi:10.1093/ofid/ofv100, Impact of Late Oseltamivir Treatment on Influenza Symptoms in the Outpatient Setting: Results of a Randomized Trial, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4525010/.
118.Medical World Nigeria, Chloroquine potent for COVID-19 prevention, says NAFDAC, https://medicalworldnigeria.com/po..9-Prevention-Says-NAFDAC?pid=45479.
119.Medical Xpress, Senegal says hydroxychloroquine virus treatment is promising, https://medicalxpress.com/news/202..xychloroquine-virus-treatment.html.
120.Medical Xpress (B), Amid global controversy, Greece moves forward with chloroquine, https://medicalxpress.com/news/202..ontroversy-greece-chloroquine.html.
121.Membrillo de Novales et al., Preprints 2020, 2020050057, doi:10.20944/preprints202005.0057.v1, Early Hydroxychloroquine Is Associated with an Increase of Survival in COVID-19 Patients: An Observational Study, https://www.preprints.org/manuscript/202005.0057.
122.Meneguesso, A., Médica defende tratamento precoce da Covid-19, https://www.youtube.com/watch?v=X5FCrIm_19U.
123.Middle East Eye, Coronavirus: Turkey says hydroxychloroquine dramatically reduces pneumonia cases, https://www.middleeasteye.net/news..roquine-malaria-treatment-progress.
124.Mikami et al., J. Gen. Intern. Med., doi:10.1007/s11606-020-05983-z, Risk Factors for Mortality in Patients with COVID-19 in New York City, https://link.springer.com/article/10.1007/s11606-020-05983-z.
125.Ministerstva Zdravotnictví, Rozhodnutí o dočasném povolení neregistrovaného humánního léčivého přípravku HYDROXYCHLOROQUINE SULFATE TABLETS, https://www.mzcr.cz/rozhodnuti-o-d..ydroxychloroquine-sulfate-tablets/.
126.Mitchell et al., SSRN, doi:10.2139/ssrn.3586954, Markedly Lower Rates of Coronavirus Infection and Fatality in Malaria-Endemic Regions – A Clue As to Treatment?, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3586954.
127.Mitjà et al., medRxiv, doi:10.1101/2020.07.20.20157651, A Cluster-Randomized Trial of Hydroxychloroquine as Prevention of Covid-19 Transmission and Disease, https://www.medrxiv.org/content/10.1101/2020.07.20.20157651v1.
128.Mitjà (B) et al., Clinical Infectious Diseases, ciaa1009, doi:10.1093/cid/ciaa1009, Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial, https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa1009/5872589.
129.Morocco World News, Moroccan Scientist: Morocco’s Chloroquine Success Reveals European Failures, https://www.moroccoworldnews.com/2..success-reveals-european-failures/.
130.Mosaique Guinee, Traitement des malades de covid19 en Guinée: « nous continuons avec l’hydroxychloroquine » (ANSS), https://mosaiqueguinee.com/traitem..ons-avec-lhydroxychloroquine-anss/.
131.Nachega et al., The American Journal of Tropical Medicine and Hygiene, doi:10.4269/ajtmh.20-1240, Clinical Characteristics and Outcomes of Patients Hospitalized for COVID-19 in Africa: Early Insights from the Democratic Republic of the Congo, https://www.ajtmh.org/content/journals/10.4269/ajtmh.20-1240.
132.Nichol et al., Injury, 2010, doi: 10.1016/j.injury.2010.03.033, Challenging issues in randomised controlled trials, https://www.injuryjournal.com/article/S0020-1383(10)00233-0/fulltext.
133.Nigeria News World, COVID-19: Jigawa govt reveals secret behind mass recovery of patients, https://nigerianewsworld.com/news/..-behind-mass-recovery-of-patients/.
134.NPR News, Senegal pledges a bed for every coronavirus patient, https://wfuv.org/content/senegal-p..t-%E2%80%94-and-their-contacts-too.
135.Oneindia, No COVID-19 death in Manipur, Mizoram, Nagaland, Sikkim so far: Govt, https://www.oneindia.com/india/no-..o-far-health-ministry-3111048.html.
136.Paccoud et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaa791, Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital, https://academic.oup.com/cid/article/doi/10.1093/cid/ciaa791/5859555.
137.Pan African Medical Journal, Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study, https://www.panafrican-med-journal.com/content/series/37/1/9/full/.
138.Parola et al., COVID-19 in Africa: What else?, https://www.mediterranee-infection..oads/2020/09/COVIDAfricaJOUMII.pdf.
139.Peters et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2020.10.004 (preprint 8/15), Outcomes of Persons With COVID-19 in Hospitals With and Without Standard Treatment With (Hydroxy)chloroquine, https://www.clinicalmicrobiologyan..cle/S1198-743X(20)30615-7/fulltext.
140.Pilot News, Chloroquine Can Treat Coronavirus at Early Stage – NAFDAC DG, https://www.westafricanpilotnews.c..onavirus-at-early-stage-nafdac-dg/.
141.Pinato et al., Cancer Discovery, doi:10.1158/2159-8290.CD-20-0773, Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients, https://cancerdiscovery.aacrjourna..ly/2020/08/18/2159-8290.CD-20-0773.
142.Pleno.News, Cuba stands out in combating Covid with hydroxychloroquine, https://pleno.news/saude/coronavir..a-covid-com-hidroxicloroquina.html.
143.Polat et al., Medical Journal of Bakirkoy, 16:3, 280-6, doi:10.5222/BMJ.2020.50469, Hydroxychloroquine Use on Healthcare Workers Exposed to COVID-19 -A Pandemic Hospital Experience, https://www.bakirkoytip.org/jvi.as..oytip&plng=eng&un=BMJ-50469&look4=.
144.Q Costa Rica, Hydroxychloroquine: The Drug Costa Rica Uses Successfully To Fight Covid-19, https://qcostarica.com/hydroxychlo..es-successfully-to-fight-covid-19/.
145.Rajasingham et al., medRxiv, doi:10.1101/2020.09.18.20197327, Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, https://academic.oup.com/cid/advan..e/doi/10.1093/cid/ciaa1571/5929230.
146.Rathi et al. Lancet Infect. Dis. doi:10.1016/S1473-3099(20)30313-3, Hydroxychloroquine prophylaxis for COVID-19 contacts in India, https://www.thelancet.com/journals../PIIS1473-3099(20)30313-3/fulltext.
147.RECOVERY Collaborative Group, NEJM, doi:10.1056/NEJMoa2022926 (press release 6/5), Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial, https://www.nejm.org/doi/full/10.1056/NEJMoa2022926.
148.Rentsch et al., medRxiv, doi:10.1101/2020.09.04.20187781, Hydroxychloroquine for prevention of COVID-19 mortality: a population-based cohort study, https://www.medrxiv.org/content/10.1101/2020.09.04.20187781v1.
149.Rivera et al., Cancer Discovery, doi:10.1158/2159-8290.CD-20-0941, Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study, https://cancerdiscovery.aacrjourna..ly/2020/09/12/2159-8290.CD-20-0941.
150.Roomi et al., J. Medical Internet Research, doi:10.2196/21758, Efficacy of hydroxychloroquine and tocilizumab in patients with COVID-19: A single-center retrospective chart review, https://www.jmir.org/2020/9/e21758/.
151.Rosenberg et al., JAMA, May 11, 2020, doi:10.1001/jama.2020.8630, Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State, https://jamanetwork.com/journals/jama/fullarticle/2766117.
152.Russian Government, Распоряжение Правительства Российской Федерации от 16.04.2020 № 1030-р, http://publication.pravo.gov.ru/Document/View/0001202004160037#print.
153.Saleemi et al., medRxiv, doi:10.1101/2020.08.05.20151027, Time to negative PCR from symptom onset in COVID-19 patients on Hydroxychloroquine and Azithromycin – A real world experience, https://www.medrxiv.org/content/10.1101/2020.08.05.20151027v1.
154.Sbidian et al., medRxiv, doi:10.1101/2020.06.16.20132597, Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France, https://www.medrxiv.org/content/10.1101/2020.06.16.20132597v1.
155.Scholz et al., Preprints 2020, 2020070025, doi:10.20944/preprints202007.0025.v1, COVID-19 Outpatients – Early Risk-Stratified Treatment with Zinc Plus Low Dose Hydroxychloroquine and Azithromycin: A Retrospective Case Series Study, https://www.preprints.org/manuscript/202007.0025/v1.
156.Serrano et al., Ann. Oncol., 2020, Sep, 31, S1026, doi:10.1016/j.annonc.2020.08.1830, COVID-19 and lung cancer: What do we know?, https://www.annalsofoncology.org/a..cle/S0923-7534(20)41826-5/fulltext.
157.Shabrawishi et al., medRxix, doi:10.1101/2020.05.08.20095679, Negative nasopharyngeal SARS-CoV-2 PCR conversion in response to different therapeutic interventions, https://www.medrxiv.org/content/10.1101/2020.05.08.20095679v1.
158.Shoaibi et al., medRxiv, doi:10.1101/2020.09.23.20199463, Comparative Effectiveness of Famotidine in Hospitalized COVID-19 Patients, https://www.medrxiv.org/content/10.1101/2020.09.23.20199463v1.
159.Singer et al., Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-218500, Hydroxychloroquine ineffective for COVID-19 prophylaxis in lupus and rheumatoid arthritis, https://ard.bmj.com/content/early/2020/08/19/annrheumdis-2020-218500.
160.Singh et al., medRxiv, doi:10.1101/2020.05.12.20099028, Outcomes of Hydroxychloroquine Treatment Among Hospitalized COVID-19 Patients in the United States- Real-World Evidence From a Federated Electronic Medical Record Network, https://www.medrxiv.org/content/10.1101/2020.05.12.20099028v1.
161.Skipper et al., Annals of Internal Medicine, doi:10.7326/M20-4207, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial, https://www.acpjournals.org/doi/10.7326/M20-4207.
162.SOLIDARITY Trial Consortium, medRxiv, doi:10.1101/2020.10.15.20209817, Repurposed antiviral drugs for COVID-19; interim WHO SOLIDARITY trial results, https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1.
163.Soto-Becerra et al., medRxiv, doi:10.1101/2020.10.06.20208066, Real-World Effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: Results of a target trial emulation using observational data from a nationwide Healthcare System in Peru, https://www.medrxiv.org/content/10.1101/2020.10.06.20208066v1.
164.Sulaiman et al., medRxiv, doi:10.1101/2020.09.09.20184143, The Effect of Early Hydroxychloroquine-based Therapy in COVID-19 Patients in Ambulatory Care Settings: A Nationwide Prospective Cohort Study, https://www.medrxiv.org/content/10.1101/2020.09.09.20184143v1.
165.Sánchez-Álvarez et al., Nefrología, doi:10.1016/j.nefroe.2020.04.002, Status of SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN), https://www.sciencedirect.com/science/article/pii/S201325142030050X.
166.Tang et al., BMJ 2020, 369, doi:10.1136/bmj.m1849, Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial, https://www.bmj.com/content/369/bmj.m1849.
167.Teller Report, Coronavirus: a study in Senegal confirms the effectiveness of hydroxychloroquine, http://www.tellerreport.com/news/2..hydroxychloroquine.BJeet4Kst8.html.
168.The Africa Report, Coronavirus: Didier Raoult the African and chloroquine, from Dakar to Brazzaville, https://www.theafricareport.com/26..roquine-from-dakar-to-brazzaville/.
169.The Australian, India and Indonesia stand by antimalarials, https://www.theaustralian.com.au/w..y/d7856d1371697fe69e4fcc39d7f1f97c.
170.The BL, Russia supports the use of hydroxychloroquine, the drug to treat the CCP Virus suggested by Trump, https://thebl.com/world-news/russi..oroquine-drug-ccp-virus-trump.html.
171.The East African, Algeria backs use of malaria drug despite WHO dropping trials, https://www.theeastafrican.co.ke/n../4552902-5564930-duphp6/index.html.
172.The Guardian, Chloroquine potent for COVID-19 prevention, says NAFDAC, https://guardian.ng/news/nigeria/n..r-covid-19-prevention-says-nafdac/.
173.The Indian Express, Vadodara administration drive: HCQ helping in containing Covid-19 cases, say docs as analysis begins, https://indianexpress.com/article/..y-docs-as-analysis-begins-6486049/.
174.The Moscow Times, Russia Approves Unproven Malaria Drug to Treat Coronavirus, https://www.themoscowtimes.com/202..a-drug-to-treat-coronavirus-a70025.
175.The New York Times, Malaria Drug Taken by Trump Is Tied to Increased Risk of Heart Problems and Death in New Study, https://www.nytimes.com/2020/05/22..alaria-drug-trump-coronavirus.html.
176.The New York Times (B), Small Chloroquine Study Halted Over Risk of Fatal Heart Complications, https://www.nytimes.com/2020/04/12..ronavirus-trump.html?smid=em-share.
177.The New York Times (C), Malaria Drug Promoted by Trump Did Not Prevent Covid Infections, Study Finds, https://www.nytimes.com/2020/06/03..chloroquine-coronavirus-trump.html.
178.The New York Times (D), Coronavirus Can Be Deadly for Young Adults, Too, Study Finds, https://www.nytimes.com/2020/09/10/world/covid-19-coronavirus.html.
179.The North Africa Post, Morocco continues use of Chloroquine despite controversy, https://northafricapost.com/41247-..loroquine-despite-controversy.html.
180.The Tico Times, News briefs: Reopening plans on-track, hydroxychloroquine use to continue, partnership with Coursera, https://ticotimes.net/2020/06/15/n..continue-partnership-with-coursera.
181.Treanor et al., JAMA, 2000, 283:8, 1016-1024, doi:10.1001/jama.283.8.1016, Efficacy and Safety of the Oral Neuraminidase Inhibitor Oseltamivir in Treating Acute Influenza: A Randomized Controlled Trial, https://jamanetwork.com/journals/jama/fullarticle/192425.
182.Ukraine Ministry of Health Care, ПРОТОКОЛ «НАДАННЯ МЕДИЧНОЇ ДОПОМОГИ ДЛЯ ЛІКУВАННЯ КОРОНАВІРУСНОЇ ХВОРОБИ (COVID-19)» , https://www.dec.gov.ua/wp-content/..04/2020_762_protokol_covid19-f.pdf.
183.Ukrinform, Ukraine receives batch of hydroxychloroquine tablets from India, https://www.ukrinform.net/rubric-e..ose-down-in-ukraine-on-june-3.html.
184.Ulrich et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaa446, Treating Covid-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind, Randomized Controlled Trial in Hospitalized Patients, https://academic.oup.com/ofid/adva..e/doi/10.1093/ofid/ofaa446/5910201.
185.Vanguard, COVID-19: Nigerian study finds Chloroquine, Hydroxychloroquine effective as Prophylaxis, https://www.vanguardngr.com/2020/0..oroquine-effective-as-prophylaxis/.
186.Voice of America, Cameroon Begins Large-scale Chloroquine Production, https://www.voanews.com/science-he..large-scale-chloroquine-production.
187.Wang et al., medRxiv, doi:10.1101/2020.06.11.20128926, Comorbidity and Sociodemographic determinants in COVID-19 Mortality in an US Urban Healthcare System, https://www.medrxiv.org/content/10.1101/2020.06.11.20128926v1.
188.Xia et al., ChiCTR2000029741, Efficacy of Chloroquine and Lopinavir/ Ritonavir in mild/general novel coronavirus (CoVID-19) infections: a prospective, open-label, multicenter randomized controlled clinical study, http://www.chictr.org.cn/showproj.aspx?proj=49263.
189.Yu et al., Science China Life Sciences, 2020 May 15, 1-7, doi:10.1007/s11427-020-1732-2, Low Dose of Hydroxychloroquine Reduces Fatality of Critically Ill Patients With COVID-19, https://pubmed.ncbi.nlm.nih.gov/32418114/.
190.Zhang et al., JAMA, 80:19, 1690, doi:10.1001/jama.280.19.1690, What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes, https://jamanetwork.com/journals/jama/fullarticle/188182.
191.Zhong et al., Lancent Rheumatology, 10.1016/S2665-9913(20)30227-7, COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study, https://www.thelancet.com/journals../PIIS2665-9913(20)30227-7/fulltext.
192.Zhong (B) Nanshan (钟南山), Efficacy and safety of chloroquine for treatment of COVID-19. An open-label, multi-center, non-randomized trial, https://twitter.com/JamesTodaroMD/status/1243260720944480265.
Appendix 1. Methods and Study Results
We performed ongoing searches of PubMed, medRxiv, ClinicalTrials.gov, The Cochrane Library, Google Scholar, Collabovid, the reference lists of other studies and meta-analyses, and submissions to the site c19study.com, which regularly receives submissions of both positive and negative studies upon publication. Search terms were hydroxychloroquine or chloroquine and COVID-19 or SARS-CoV-2, or simply hydroxychloroquine or chloroquine. All studies regarding the use of HCQ or CQ for COVID-19 that report an effect compared to a control group are included in the main analysis. This is a living analysis and will be updated regularly.
We extracted effect sizes and associated data from all studies. If studies report multiple kinds of effects then the most serious outcome is used in calculations for that study. For example, if effects for deaths and cases are both reported, the effect for death is used, this may be different to the effect that a study focused on. If mortality results are given at multiple times, we used the longest time. Clinical outcome is considered more important than PCR testing status. When results provide an odds ratio, we computed the relative risk when possible, or converted to a relative risk according to [Zhang]. Reported confidence intervals and p-values were used when available, using adjusted values when provided. When needed, conversion between reported p-values and confidence intervals followed [Altman, Altman (B)], and Fisher’s exact test was used to calculate p-values for event data. If a study separated HCQ and HCQ+AZ we used the combined results were possible, or the results for the larger group. Results are all expressed with RR < 1.0 suggesting effectiveness. Most results are the relative risk of something negative. A few studies report relative times, where the results are expressed as the ratio of the time for the HCQ group versus the time for the control group. One study reports the rate of reduction of viral load, where the result is based on the percentage change in the rate.
Calculations were done in Python (3.8.5) with scipy (1.3.3), pythonmeta (1.11), numpy (1.19.1), statsmodels (0.12.0), and plotly (4.10.0). The forest plot is computed using PythonMeta [Deng] with the DerSimonian and Laird random effects model (the fixed effect assumption is not plausible in this case). We received no funding, this research is done in our spare time. We have no affiliations with any pharmaceutical companies or political parties.For early treatment, we have used a cutoff of 5 days after symptoms, although a shorter time may be preferable. Antivirals are typically only considered effective when used within a shorter timeframe, for example 0-36 or 0-48 hours for oseltamivir, with longer delays not being effective [McLean, Treanor].A summary of study results is below. It is easy to propose excluding certain papers for various reasons, for example [Fried, Kelly, Kuderer, McGrail] report negative results but do not themselves consider the results comparable – they note that treated patients were significantly more ill and do not make adjustments.
To avoid potential bias in evaluation we currently include all studies. HCQ research exhibits a negative bias as shown above and addressing this bias will increase the observed efficacy. Given the state of scientific discussion about HCQ, we feel that a conservative approach is appropriate, especially since efficacy is clear even with this approach. For reference, a draft analysis excluding studies with major issues can be found in Appendix 2.
Please submit updates and corrections with the form at the bottom of this page.Pre‑Exposure Prophylaxis[Abella], risk of COVID-19 case, RR 0.95, p = 1.00.[Bhattacharya], risk of COVID-19 case, RR 0.19, p = 0.001.[Cassione], risk of COVID-19 case, RR 1.50, p = 0.59.[Chatterjee], full course vs. unused risk of COVID-19 case, RR 0.33, p < 0.001.[de la Iglesia], risk of hospitalization, RR 1.50, p = 1.00.[de la Iglesia], suspected COVID-19, RR 1.43, p = 0.15.[de la Iglesia], confirmed COVID-19, RR 0.92, p = 0.84.[Ferreira], risk of COVID-19 case, RR 0.53, p < 0.001.[Ferri], risk of COVID-19 case, RR 0.37, p = 0.01.[Gendebien], risk of COVID-19 case, RR 0.96, p = 0.93.[Gendelman], risk of COVID-19 case, RR 0.92, p = 0.88.[Gentry], risk of death, RR 0.13, p = 0.10.[Gentry], risk of COVID-19 case, RR 0.79, p = 0.27.[Gianfrancesco], risk of hospitalization, RR 0.97, p = 0.82.[Grau-Pujol], risk of COVID-19 case, RR 0.30, p = 0.47.[Huang], risk of hospitalization, RR 0.20, p < 0.001.[Huh], risk of COVID-19 case, RR 1.48, p = 0.09.[Khurana], risk of COVID-19 case, RR 0.49, p = 0.02.[Konig], risk of hospitalization, RR 0.97, p = 0.88.[Laplana], risk of COVID-19 case, RR 1.56, p = 0.24.[Macias], risk of hospitalization, RR 0.74, p = 1.00.[Macias], risk of COVID-19 case, RR 1.49, p = 0.53.[Mitchell], risk of death, RR 0.01, p < 0.001.[Rajasingham], risk of hospitalization, RR 0.50, p = 1.00.[Rajasingham], risk of COVID-19 case, RR 0.73, p = 0.12.[Rentsch], risk of death, RR 1.03, p = 0.83.[Singer], risk of COVID-19 case, RR 1.09, p = 0.62.[Zhong], risk of COVID-19 case, RR 0.09, p = 0.04.Post‑Exposure Prophylaxis[Boulware (B)], risk of COVID-19 case, RR 0.83, p = 0.35.[Mitjà], risk of death, RR 0.68, p = 0.58.[Mitjà], baseline pcr- risk of cases, RR 0.70, p = 0.15.[Polat], risk of COVID-19 case, RR 0.43, p = 0.03.Early treatment[Ashraf], risk of death, RR 0.32, p = 0.15.[Chen], median time to PCR-, RR 0.28, p = 0.01.[Esper], risk of hospitalization, RR 0.36, p = 0.02.[Gautret], risk of no virological cure at day 6, RR 0.34, p = 0.001.[Guisado-Vasco], risk of death, RR 0.12, p = 0.001.[Guérin], risk of death, RR 0.57, p = 0.73.[Guérin], risk of no recovery, RR 0.35, p < 0.001.[Heras], risk of death, RR 0.08, p < 0.001.[Hong], risk of prolonged viral shedding, RR 0.35, p = 0.001.[Huang (B)], risk of no virological cure, RR 0.41, p < 0.001.[Huang (C)], risk of no recovery at day 14, RR 0.09, p = 0.09.[Huang (C)], risk of no improvement in pneumonia at day 14, RR 0.17, p = 0.22.[Ip], risk of hospitalization, RR 0.54, p = 0.03.[Izoulet], risk of death, RR 0.15, p < 0.001.[Kirenga], median time to recovery, RR 0.74, p = 0.20.[Lagier], risk of death, RR 0.41, p = 0.05.[Ly], risk of death, RR 0.46, p = 0.03.[Mitjà (B)], risk of hospitalization, RR 0.75, p = 0.64.[Mitjà (B)], risk of no recovery, RR 0.83, p = 0.38.[Scholz], risk of death, RR 0.21, p = 0.13.[Scholz], risk of hospitalization, RR 0.18, p < 0.001.[Skipper], risk of hospitalization, RR 0.60, p = 0.17.[Skipper], risk of no recovery at day 14, RR 0.80, p = 0.21.[Sulaiman], risk of death, RR 0.36, p = 0.01.[Sulaiman], risk of hospitalization, RR 0.61, p = 0.001.Late treatment[Abd-Elsalam], risk of death, RR 1.20, p = 1.00.[Abd-Elsalam], risk of no recovery at day 28, RR 0.70, p = 0.009.[Alamdari], risk of death, RR 0.45, p = 0.03.[Alberici], risk of death, RR 0.57, p = 0.12.[Almazrou], risk of ventilation, RR 0.35, p = 0.16.[Almazrou], risk of ICU admission, RR 0.79, p = 0.78.[An], time to viral clearance, RR 0.97, p = 0.92.[Annie], risk of death, RR 0.96, p = 0.83.[Annie], risk of death, RR 1.21, p = 0.46.[Aparisi], risk of death, RR 0.37, p = 0.008.[Arshad], risk of death, RR 0.49, p = 0.009.[Ashinyo], risk of hospitalization, RR 0.67, p = 0.03.[Ayerbe], risk of death, RR 0.48, p < 0.001.[Barbosa], risk of death, RR 2.47, p = 0.58.[Bernaola], risk of death, RR 0.83, p < 0.001.[Bousquet], risk of death, RR 0.57, p = 0.15.[Catteau], risk of death, RR 0.68, p < 0.001.[Cavalcanti], HCQ+HCQ/AZ risk of death, RR 0.84, p = 0.77.[Cavalcanti], HCQ+HCQ/AZ risk of hospitalization, RR 1.28, p = 0.30.[Chen (B)], risk of no virological cure, RR 0.76, p = 0.71.[Chen (B)], median time to PCR-, RR 0.50, p = 0.40.[Chen (C)], risk of no virological cure, RR 1.29, p = 0.70.[Chen (D)], risk of no improvement in pneumonia at day 6, RR 0.43, p = 0.04.[Chen (E)], risk of radiological progression, RR 0.71, p = 0.57.[Chen (E)], risk of viral+ at day 7, RR 2.00, p = 1.00.[Cravedi], risk of death, RR 1.53, p = 0.17.[D’Arminio Monforte], risk of death, RR 0.66, p = 0.12.[Davido], risk of combined intubation/hospitalization, RR 0.45, p = 0.04.[Di Castelnuovo], risk of death, RR 0.70, p < 0.001.[DISCOVERY], 29 day mortality estimated from graph, RR 0.69, p = 0.35.[DISCOVERY], risk of 7-point scale status, RR 0.83, p = 0.40.[Dubernet], risk of ICU admission, RR 0.12, p = 0.008.[Fontana], risk of death, RR 0.50, p = 0.53.[Fried], risk of death, RR 1.27, p < 0.001.[Geleris], risk of combined intubation/death, RR 1.04, p = 0.76.[Goldman], risk of death, RR 0.78, p = 0.46.[Gonzalez], risk of death, RR 0.73, p = 0.06.[Guisado-Vasco (B)], risk of death, RR 0.80, p = 0.36.[Gupta], risk of death, RR 1.06, p = 0.41.[Heberto], risk of death, RR 0.46, p = 0.04.[Heberto], risk of ventilation, RR 0.34, p = 0.008.[Huang (D)], risk of no virological cure, RR 0.33, p < 0.001.[Ip (B)], risk of death, RR 0.99, p = 0.93.[Kamran], risk of disease progression, RR 0.95, p = 1.00.[Kamran], risk of viral+ at day 14, RR 1.10, p = 0.52.[Kelly], risk of death, RR 2.43, p = 0.03.[Kim], risk of hospitalization, RR 0.49, p = 0.01.[Kim], risk of no virological cure, RR 0.44, p = 0.005.[Kuderer], risk of death, RR 2.34, p < 0.001.[Lammers], risk of combined death/ICU, RR 0.68, p = 0.02.[Lauriola], risk of death, RR 0.27, p < 0.001.[Lecronier], risk of death, RR 0.58, p = 0.24, HCQ vs. control.[Lecronier], risk of treatment escalation, RR 0.94, p = 0.73, HCQ vs. control.[Lecronier], risk of viral+ at day 7, RR 0.85, p = 0.61, HCQ vs. control.[Luo], risk of death, RR 1.02, p = 0.99.[Lyngbakken], improvement in viral load reduction rate, RR 0.29, p = 0.51.[Magagnoli], risk of death, RR 1.31, p = 0.28.[Mahévas], risk of death, RR 1.20, p = 0.75.[McGrail], risk of death, RR 1.70, p = 0.69.[Membrillo de Novales], risk of death, RR 0.45, p = 0.002.[Mikami], risk of death, RR 0.53, p < 0.001.[Nachega], risk of death, RR 0.72, p = 0.17.[Nachega], risk of no improvement, RR 0.74, p = 0.13.[Paccoud], risk of death, RR 0.89, p = 0.88.[Peters], risk of death, RR 1.09, p = 0.57.[Pinato], risk of death, RR 0.41, p < 0.001.[RECOVERY], risk of death, RR 1.09, p = 0.15.[Rivera], risk of death, RR 1.02, p = 0.90.[Roomi], risk of death, RR 1.38, p = 0.54.[Rosenberg], risk of death, RR 1.35, p = 0.31.[Saleemi], median time to PCR-, RR 1.21, p < 0.05.[Sbidian], risk of death, RR 1.05, p = 0.74, whole population HCQ AIPTW adjusted.[Sbidian], risk of no hospital discharge, RR 0.80, p = 0.002, whole population HCQ AIPTW adjusted.[Serrano], risk of death, RR 0.57, p = 0.14.[Shabrawishi], risk of no virological cure at day 5, RR 0.85, p = 0.66.[Shoaibi], risk of death, RR 0.85, p < 0.001.[Singh], risk of death, RR 0.95, p = 0.72.[Singh], risk of ventilation, RR 0.81, p = 0.26.[SOLIDARITY], risk of death, RR 1.19, p = 0.23.[Soto-Becerra], risk of death, RR 1.84, p = 0.02.[Sánchez-Álvarez], risk of death, RR 0.54, p = 0.005.[Tang], risk of no virological cure at day 21, RR 0.79, p = 0.51.[Ulrich], risk of death, RR 1.06, p = 1.00.[Wang], risk of death, RR 0.94, p = 0.63.[Xia], risk of no virological cure, RR 0.62, p = 0.17.[Yu], risk of death, RR 0.40, p = 0.002.[Zhong (B)], risk of no virological cure at day 10, RR 0.20, p < 0.001.
Appendix 2. Draft Analysis with Exclusions
Many meta-analyses for HCQ have been written, most of which have become somewhat obselete due to the continuing stream of more recent studies. Recent analyses with positive conclusions include [IHU Marseille] which considers significant bias from an understanding of each trial, and [Garcia-Albeniz, Ladapo, Prodromos] which focus on early or prophylactic use studies.Meta analyses reporting negative conclusions focus on late treatment studies, tend to disregard treatment delay, tend to follow formulaic evaluations which overlook major issues with various studies, and end up with weighting disproportionate to a reasoned analysis of each study’s contribution. For example, [Axfors] assigns 87% weight to a single trial, the RECOVERY trial [RECOVERY], thereby producing the same result. However, the RECOVERY trial may be the most biased of the studies they included, due to the excessive dosage used, close to the level shown to be very dangerous in [Borba] (OR 2.8), and with extremely sick late stage patients (60% requiring oxygen, 17% ventilation/ECMO, and a very high mortality rate in both arms). There is little reason to suggest that the results from this trial are applicable to more typical dosages or to earlier treatment.We include all studies in the main analysis, however there are major issues with several studies that could significantly alter the results. Here, we present a draft analysis excluding studies with significant issues, including indication of significant unadjusted group differences or confouding by indication, extremely late stage usage >14 days post symptoms or >50% on oxygen at baseline, very minimal detail provided, excessive dosages which have been shown to be dangerous, significant issues with adjustments that could reasonably make substantial differences, and reliance on PCR which may be inaccurate and less indicative of severity than symptoms. We welcome feedback on improvements or corrections to this. The studies excluded are as follows, and the resulting forest plot is shown in Figure 10.[Alamdari], substantial unadjusted confounding by indication.[An], results only for PCR status which may be significantly different to symptoms.[Annie], confounding by indication is likely and adjustments do not consider COVID-19 severity.[Barbosa], excessive unadjusted differences between groups.[Cassione], not fully adjusting for the different baseline risk of systemic autoimmune patients.[Chen], results only for PCR status which may be significantly different to symptoms.[Chen (B)], results only for PCR status which may be significantly different to symptoms.[Chen (C)], results only for PCR status which may be significantly different to symptoms.[Cravedi], substantial unadjusted confounding by indication.[de la Iglesia], not fully adjusting for the different baseline risk of systemic autoimmune patients.[Fried], excessive unadjusted differences between groups, substantial unadjusted confounding by indication.[Gautret], excessive unadjusted differences between groups, results only for PCR status which may be significantly different to symptoms.[Geleris], significant issues found with adjustments.[Gendebien], not fully adjusting for the baseline risk differences within systemic autoimmune patients.[Gendelman], not fully adjusting for the different baseline risk of systemic autoimmune patients.[Gianfrancesco], not fully adjusting for the baseline risk differences within systemic autoimmune patients.[Gupta], >50% on oxygen/ventilation at baseline.[Hong], results only for PCR status which may be significantly different to symptoms.[Huang], significant unadjusted confounding possible.[Huang (B)], results only for PCR status which may be significantly different to symptoms.[Huang (D)], results only for PCR status which may be significantly different to symptoms.[Huh], not fully adjusting for the different baseline risk of systemic autoimmune patients.[Izoulet], excessive unadjusted differences between groups.[Kamran], excessive unadjusted differences between groups.[Kelly], substantial unadjusted confounding by indication.[Konig], not fully adjusting for the baseline risk differences within systemic autoimmune patients.[Kuderer], substantial unadjusted confounding by indication.[Laplana], not fully adjusting for the different baseline risk of systemic autoimmune patients.[Lecronier], >50% on oxygen/ventilation at baseline.[Luo], substantial unadjusted confounding by indication.[Lyngbakken], results only for PCR status which may be significantly different to symptoms.[Macias], not fully adjusting for the baseline risk differences within systemic autoimmune patients.[McGrail], excessive unadjusted differences between groups.[Mitchell], excessive unadjusted differences between groups.[Peters], excessive unadjusted differences between groups.[RECOVERY], excessive dosage, results do not apply to typical dosages.[Rentsch], not fully adjusting for the baseline risk differences within systemic autoimmune patients, medication adherence unknown and may significantly change results.[Roomi], substantial unadjusted confounding by indication.[Saleemi], results only for PCR status which may be significantly different to symptoms, substantial unadjusted confounding by indication.[Sbidian], significant issues found with adjustments.[Shabrawishi], results only for PCR status which may be significantly different to symptoms.[Singer], not fully adjusting for the baseline risk differences within systemic autoimmune patients.[Singh], confounding by indication is likely and adjustments do not consider COVID-19 severity.[SOLIDARITY], excessive dosage, results do not apply to typical dosages, >50% on oxygen/ventilation at baseline.[Soto-Becerra], confounding by indication is likely and adjustments do not consider COVID-19 severity.[Tang], results only for PCR status which may be significantly different to symptoms.[Ulrich], >50% on oxygen/ventilation at baseline.[Wang], confounding by indication is likely and adjustments do not consider COVID-19 severity.[Xia], detail too minimal.[Zhong (B)], results only for PCR status which may be significantly different to symptoms.
Related: