Source: France Soir
Italy: December 7 the teachers: Prof. Alessandro Capucci, cardiologist, Prof. Luigi Cavanna, oncologist and Dr. Paola Varese have provided a medical scientific report on the use of hydroxychloroquine in the first symptoms of sras-cov2 pathology .
This report forms the scientific basis, the arguments of which were taken up by the Italian Council of State in the decision for the authorization of hydroxychloroquine in the treatment of Covid-19 in the early phase.
After an introduction, he makes a complete review of the evaluation of the data available according to the rules of evidence-based medicine: internal validity and external validity, the data available on hydroxychloroquine (HCQ), studies on the use of HCQ in early-stage COVID-19 disease, and statistical testing of HCQ observational data in early therapy. It also incorporates the studies cited by the Italian National Health Agency (AIFA), taking into account pharmacological considerations and the safety of the drug. In the second part, he presents a final critique and the distribution of responsibilities in the health system. Before concluding, this report establishes the asymmetry of information that exists among regulators and conflicts of interest in science and among authorities that affect research ethics.
Prof. Alessandro Capucci is the former director of the School of Specialization in Cardiovascular Diseases at the Polytechnic University of Marche and Director of the Cardiology Clinic at Torrette Hospital in Ancona, former Director of the Schools of Specialized Thoracic Surgery and Surgery vascular. For his part, Prof. Luigi Cavanna, the oncologist is director of the Department of Oncology-Hematology. USL company in Piacenza. And Dr. Paola Varese is the director of SC Medicine and DH oncology ASLAL Piemont.
Conclusions: the 30-page report is presented below, the conclusions of which were taken up by the Italian Council of State.
As a result of a current major infectious event like Covid 19, its rapid spread, the devastating impact on hospital and territorial health establishments, regulatory bodies, international research networks (Cochrane in the first place) will not produce guidelines and timely information to guide the care of large numbers of patients who become ill, as is often the case with infectious diseases that spread very quickly.
The evidence presented to support the IAAF decision of its decision is in most cases drawn from hospital care settings, or even intensive care settings, where the difficulty of obtaining relevant clinical improvements in patients. patients with non-systemic impairment are evident.
It is paradoxical that in view of these results, considered discouraging by the AIFA, it decides to limit the use of HCQ in clinical trials, which in fact amounts to limiting its use in hospitals. On the other hand, it is quite clear that if hospital mortality is that of the last few days (around 900 deaths per day), it is clear that hospital stays are not sufficient to stem the pandemic.
Faced with more than six months of experience with this disease, several published “guidelines” strongly advised against (including through legal decrees) any home treatment , as was implemented instead during the first wave of the pandemic.
From the analysis of the articles presented, it is clear that patients treated late have a worse prognosis, because if measures are not taken quickly, the viral infection triggers the cytokine storm, with potentially fatal autoimmune mechanisms. . The high mortality of COVID patients in recent periods is likely linked to a lack of early treatment of the disease.
At this stage of the pandemic, we must allow the possibility of treating patients at home, by promoting clinical diagnosis, with drugs of which we have sufficient knowledge for widespread and territorial use. It should also be taken into account that in these patients, hospitalization in itself represents a risk factor for mortality regardless of the causes of hospitalization (intercurrent hospital infections, aspiration pneumonia, difficulty in following disoriented, confused patients, hypoxemic …).
In this perspective, it should also be considered that for decades the use of HCQ has been authorized and safely recommended as a prophylaxis of malaria – even for prolonged periods – in healthy people, who take it without supervision. medical; or as continuous therapy for chronic pathologies, such as rheumatoid arthritis and lupus erythematosus, and instead, it is to be avoided for a pathology which, left alone, has a fatal outcome and for treatment lasting up to ‘to 10 days can favorably modify the fate of the disease.
A special mention for the Collectif Citoyen de FranceSoir which has worked tirelessly since March to decipher information and studies. Many comments are taken from their work.
MEDICAL SCIENTIFIC REPORT ON THE USE OF HYDROXYCHLOROQUIN AT THE EARLY SYMPTOMS OF SARS-COV2 PATHOLOGY
0 Introduction
1 Methodological plan. Evaluation of the data available according to the canons of evidence-based medicine: internal validity and external validity
1.1 Available data on hydroxychloroquine (HCQ)
1.2 Studies on the early use of HCQ in COVID-19 pathology
1.3 Binomial tests on HCQ observational data in early therapy
1.4 Studies cited by the IAAF
1.5 Pharmacological considerations
1.6 Drug safety
2. Decision-making plan
2.1 Clinical judgment and division of skills in the health system
3. Strategic plan. Research ethics, conflicts of interest and information asymmetries
4. Conclusions
Introduction
The IAAF’s decision to ban the use of hydroxychloroquine for COVID-19 therapy outside of clinical trials is inexplicable, both given the canons of evidence-based medicine (EBM) and decision-making rules ( such as that of the precautionary principle) and ethical rules, such as that of the decision-making autonomy of the doctor. In addition, the complex system of institutional mechanisms and incentives in which evidence is produced, reviewed and evaluated in the medical sector by different actors in question (pharmaceutical companies, general practitioners, monitoring agencies, academia) must be taken into account. consideration, in order to be used as a knowledge base of the regulatory and public decision-making process. To this end,
All in all, these three complementary and intersecting parts, might well help explain the situation by explaining that this is the typical catch result of the regulator. Finally, we consider, alongside these methodological evaluations, the pandemic emergency context, deaths, with psychological and media emergencies and high expectations for a new treatment.
We take the liberty of proposing simple but clear policy implications in order to strengthen the decision-making process and remove the above-mentioned distortions. The magnitude of the problem is set out below.
1 Methodological plan. Evaluating available data according to the canons of evidence-based medicine: internal validity and external validity.
Hierarchies of evidence have been developed to help healthcare professionals navigate the vastness of the information they receive from various sources and filter it based on criteria of reliability, relevance and accuracy. . According to these criteria, experimental studies, isolating the causal relationship studied from other possible explanations in depth (i.e., alternative explanations of the observed effect), are preferred to observational studies.
The latter are privileged over the series of cases without control because they make it possible to compare what happens to patients who have received and two who have not received treatment and allow first of all to check if this contributes, at least prima facie, to an improvement in the clinical image. Internally, this property ensures that the effect observed in the study is attributable to the treatment and only to the treatment, that is, there is no other explanation for the observed phenomenon.
Unsystematic meta-analyzes increase the accuracy of the measure of effect (reduced confidence interval) and systematic reviews ensure that included studies come from a representative sample of all available studies ( precision in the sense of excluding systematic distortion of the sample: no bias).
The following diagram illustrates the methodological rationale behind the hierarchy of evidence:
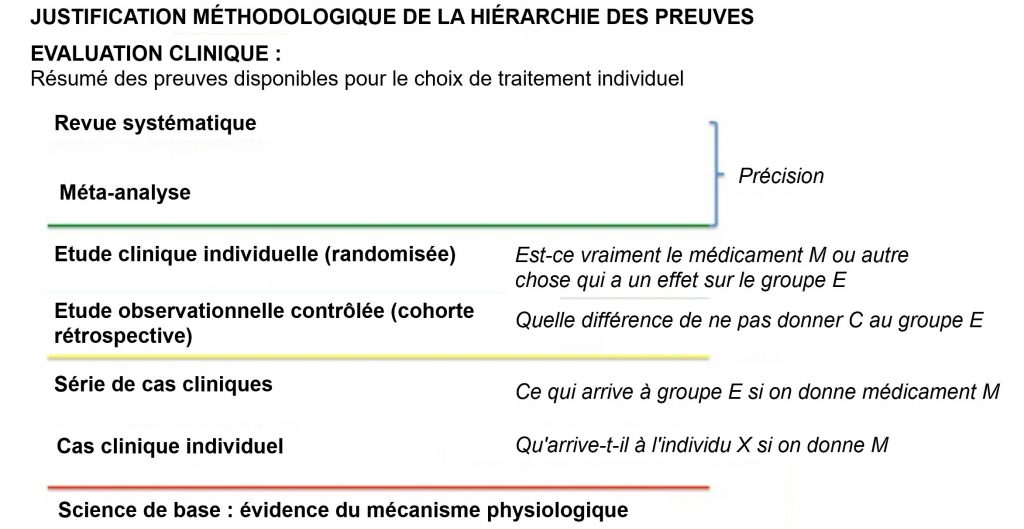
1. EBM hierarchy of evidence criteria: internal validity, external validity and accuracy.
The external validity is established from time to time by comparing the type of sample to the target population, that is to say the patient population to which the treatment is intended (EMBC 2020; GRADE 2013). The external validity of the study is essential so that it can be used to project the results on the reference population. An accurate and reliable study from the point of view of internal validity, which is not valid externally, does not meet the research demand and should be considered irrelevant, if not futile.
A non-experimental study, but carried out on the reference population, is first of all a study which provides useful information in order to establish the efficacy and safety of the drug for this type of population.
If it is not possible to exclude the “bias factors”, usually associated with non-experimental studies, or at least to consider these factors as negligible compared to the size of the observed effect – that is – i.e. if hypothetical bias factors fail to explain the observed effect (Glasziou et al. 2007, Aronson and Hauben, 2006, Hauben and Aronson 2007) the only remaining explanation is that the drug is effective, although the study is observational .
In fact, the fundamental question underlying the EBM criteria is: “What can explain the effect that I observe?” ; if I can rule out the case (random or measurement error) and there are no plausible alternatives, in terms of other causal factors or bias, which may explain the magnitude of the observed effect, the the only possible explanation that remains is that this is due to the factor studied.
The case of hydroxychloroquine reflects this species paradigm. Evidence from the field, which is the most important from the point of view of external validity – also guaranteed by correct therapeutic treatment under strict medical supervision – demonstrates with great statistical importance the efficacy and safety of the drug ( see Articles 1.3 and 1.4). In the case of uncontrolled observational studies, the benchmark to assess the size of the effect in comparative terms can obviously be derived from the incidence rate of the end point considered (e.g. hospitalization rate) in the study sample, compared to the population subjected to other treatment protocols (what is called standard care or “standard of care” SoC).
The efficacy and safety of HCQ (especially in early treatment and of limited duration) is also supported by knowledge of the pharmacokinetics of the molecule and by previous studies conducted on a similar virus, SARS-CoV-1 .
Rolain et al. (2007) provide a review of the safety profile of hydroxychloroquine for its use against SARS infection and VOCs with positive results; the United States National Institute of Health published a study in the journal Virology where it describes the drug as an active inhibitor of infection with CORONAVIRUS SARS (a potent inhibitor of SARS coronavirus infection; Vincent et coll. in 2005).
1.1 Data available on hydroxychloroquine (HCQ)
The cases collected spontaneously in various regions of Italy during the first epidemic phase (March-April 2020) show consistently and repeatedly that the comparison between the hospitalization rate of people treated with hydroxychloroquine (± azithromycin) is 5 to 6%, compared to a relative rate of 20% of subjects with SARS-COV-2 subjected to other treatment protocols, as reported by ISS reports for the same period (ISS Institut Supérieur health). Updated with the covid-19 outbreak on March 26, 2020 – 4 p.m.).
Circumstantial considerations rule out that other factors may explain the magnitude of this difference in a reproducible manner in a series of independent cases, as this is constantly noted in different situations, conducted by different physicians in different parts. from Italy (and in various regions of the world), with the only common element: the same treatment protocol.
The placebo effect, an essentially psychosomatic effect, is also insufficient to explain the difference in the evolution of the pathology in the population of treated with HCQ compared to the clinical picture of those who are not subjected to any treatment, in particular in the face of objective data such as saturation measurement and body temperature.
From the EBM’s point of view, it is incorrect to overlook the only valid outside studies on the issue, without giving valid reasons – in this case without statistically explaining the role that the in-depth funds would have in explaining the outcome. got.
Probably overzealous, the IAAF, as well as other international drug agencies and supranational institutes (FDA, EMA, WHO, etc.) responsible for safeguarding the health and well-being of citizens, have likely been overwhelmed by the considerable amount of information received with information asymmetry vis-à-vis the main knowledge producers in this sector – i.e. the pharmaceutical companies themselves, and the research institutes funded by them – considered important to examine the evidence provided to them, to prioritize the test of internal validity over that of external validity and, ultimately,to base their judgment on data that were absolutely irrelevant to elucidating the question of the efficacy and safety of HCQin home care of the first stage of SARS-CoV-2 infection .
In fact, the evidence considered, although experimental, was carried out mainly on patients at an already advanced stage and in any case already hospitalized; or, on the other end of the pathological spectrum, they are studies concerning the preventive prophylaxis of SARS-CoV-2 infection; different role from therapy.
Hydroxychloroquine can play an effective therapeutic role, especially at an early stage of the disease with a maximum activity window between zero and six days of symptom onset, and maximum on the second day (Tarek and Savarino, 2020) (Note 1). Patients who are at different stages of this time window will find it difficult to benefit from the administration of the drug at an advanced stage, as HCQ no longer has a role to play because HCQ was not able to play its role. in the early phase. In the first case, the clinical condition of the patients worsens and in the second they recover, regardless of taking HCQ.
The fact that studies carried out on asymptomatic patients exposed to COVID (but not necessarily already infected) do not show any efficacy of HCQ in terms of prophylaxis and that studies carried out on patients with advanced stage of the pathology do not show any difference in the criteria assessment of both groups is a proven fact, but at the same time irrelevant for the assessment of the relevance and adequacy of the proposed therapeutic framework.
By promoting internal validity requirements, pharmaceutical agencies have been forced, due to the lack of experimental studies conducted on the actual reference population (covid-positive patients treated at home), to violate the external validity requirement. , which precedes any other criterion, however , because it determines the relevance of the evidence .
From a legal standpoint this demonstrates its admissibility. This violation is confirmed by the use of inadequate endpoints, such as death rate or length of hospital stay, when the most appropriate endpoint to verify clinical (and pharmaco-economic) utility ) of hydroxychloroquine is that of the hospitalization rate of treated subjects .
A separate chapter would be needed to address the issue of analysis of point and flawed data from some of the studies used by IAAF and the various agencies mentioned above. We will discuss this by approaching the individual analysis of the studies cited by the IAAF in support of its decision (article 1.6).
1.2 Studies on the early use of HCQ in COVID-19 pathology
1.2.1 Gautret et al. 2020:study carried out on a small number of patients (N = 42), but with such a large effect that it provides a high rate of importance of information and data: a 50 times greater advantage of HCQ + AZ therapy ( azithromycin) compared to the standard of care (SoC) with a p value = 0.0007 (editor’s note: the closer the p is to 0, the more the statistical power is important and significant). A reanalysis performed by Risch (Lover et al., 2020) showed that the magnitude of the effect was significantly greater for the subset of asymptomatic or patients with upper respiratory tract infection compared to patients with lower respiratory tract infections. This result further confirms the specific therapeutic action of HCQ in the early treatment of COVID-19.
1.2.2 Million et al. 2020: Retrospective study by Prof. Didier Raoul with HCQ and AZ at an early stage, involving a sample of 1061 Covid-19 patients positive patients treated with HCQ + AZ for at least three days with a follow-up of at least 9 days: 973 patients cured, 88 recovering and 5 deaths. No cardiotoxicity was observed: In 973 (91.7%) patients you achieved a good clinical response and viral clearance within 10 days. The statistical analysis of these data by binomial test is shown in Table 2. (see Sec. 1.3).
1.2.3 Zelenko et al. 2020: 405 patients treated with HCQ + AZ and Zinc: 398 cured, 6 hospitalized, 2 deceased. The statistical analysis of these data by binomial test is shown in Table 2. (see Sec. 1.3).
1.2.4 Barbosa et al. 2020:Non-randomized controlled study on a sample of 636 asymptomatic subjects at high risk of infection with SARS-CoV-2 informed about the benefits and risk of treatment HCQ + AZ: 412 accepted and joined the treatment group, the other 212 joined the control group. The hospitalization rate for the treatment group was significantly lower (1.2% for patients who started treatment before 7 days after symptom onset and 3.2% for patients who started after symptoms. against 5.4% of the control group). No cardio toxic effects were found in the sample and the most common side effects in the treatment group were diarrhea (16.5%), taking into account that 12.9% were already affected before treatment. join the study.
1.2.5 Eyewitness 2020: this is a study that is still ongoing and not yet significant due to its small sample. So far, the most important data relates to the safety of the drug: no cardiotoxicity effect or other serious side effects attributable to the drug.
1.2.6 Huang et al. 2020 is a study with a small number of patients (22), whose SARS-CoV2 viral clearance was evaluated in two groups: treated with HCQ or with lopinavir / ritonavir.
The reduction in viral load that occurs before with HCQ does not translate into an immediate clinical benefit, but the pulmonary situation improves significantly earlier in affected patients treated with HCQ, as well as hospitalization times that were reduced. The authors conclude with a pragmatic assessment: in the absence of a specific treatment, old drugs like chloroquine can be relaunched to fight this new disease and save lives.
1.2.7 Ladapo et al. 2020: Meta-analysis of 5577 patients included in 5 randomized trials. The use of the drug HCQ reduces the risk of infection, hospitalization or death by 24%, with no cardiological toxicity other than gastrointestinal toxicity. No interruption of therapy due to toxicity.
1.2.8 Capucci et al. 2020: Prospective study conducted on 350 patients including 274 treated with HCQ and 76 patients with the combination HCQ + AZ early at home by MMG. No cardiac toxicity detected, hospitalization rate 5.8% and 5.2% respectively. The study presents data that follow the observational studies carried out in total emergency in Oveda (Dr Paola Varese), Piacenza (Prof. Luigi Cavanna) and Milan (Dr Andrea Mangiagalli): different populations, different social protection contexts, areas where the mortality and hospitalization rate is very high in untreated patients. The statistical analysis of these data by binomial test is shown in Table 2. (see Sec. 1.3).
1.2.9 Studies that emerged from the experience of Piacenza in Covid19 patients. 2 studies have been published on cancer patients and Covid-19, as we know and as you can guess cancer patients are a fragile subgroup of patients. The results of HCQ therapy on the first group of 25 patients were published in the journal Future Oncology (Stroppa et al. 2020): in these patients the prognosis for Covid was better with HCQ + antiretroviral treatment. The second study (Cavanna et al. 2020) includes 51 cancer patients with Covid-19; the results of this study show that the prognosis is better if the treatment based on hydroxychloroquine is started early. A third research work carried out on 124 outpatients treated with HCQ. The study includes the first month of activity in outpatient clinics; in the sample, there were no deaths and the hospital admission rate was less than 4%. These works will be dispatched within a few days for evaluation by publication. 200 other patients are then analyzed, always followed on an outpatient basis at home; even among these, no deaths and hospitalization rates of less than 5%. The statistical analysis of some of these data by binomial test is shown in Table 2. (see Sec. 1.3). even among these, no deaths and hospitalization rates of less than 5%. The statistical analysis of some of these data by binomial test is shown in Table 2. (see Sec. 1.3). even among these, no deaths and hospitalization rates of less than 5%. The statistical analysis of some of these data by binomial test is shown in Table 2. (see Sec. 1.3).
1.2.10 Yang et al. 2020: secondary analysis of data provided by the Boulware et al. In which it is observed (contrary to the conclusions of Boulware et al.), That the early treatment with HCQ reaches statistical significance (p value 0.0496). Another study reviewed by Yang et al. is that of Mitja et al. 2020 which demonstrated the formation of 55.6% more antibodies against the virus in the 14th in treated patients than in the control group. The review ends with an advantage in favor of HCQ in early administration. No major side effects were reported in the studies analyzed.
1.2.11 Cassone et al. 2020: the meta-analysis is signed by Antonio Cassone (former director of the ISS infectious diseases), Roberto Cauda (director of infectious diseases of -Gemelli, from Rome) and Licia Iacoviello (director of the department of epidemiology and prevention of Neuromed). In this meta-analysis, there is a reduction in mortality of up to 35% in patients treated with HCQ(low dose (max 400 mg / day, maximum total treatment 4500 mg, the more reduction in mortality decreases). The meta-analysis was carried out to assess the association between hydroxychloroquine (HCQ), with or without azithromycin (AZM ), and total mortality in covid-19 patients. A total of 26 scientific studies were included, for a total of 44,521 patients involved, including 7,324 patients from 4 randomized clinical trials (RCTs). Of the 26 studies, 10 were devoted to the combination HCQ and AZM. The positive effect of this dual therapy disappeared when a daily intake greater than 400 mg or a total dose greater than 4,400 mg (all treatments) were used respectively. According to the meta -analysis, the efficacy of the drug, depends on the dosage: if the 26 studies exclude the 4 randomized publications,there is a reduction in mortality of up to 35% . The effect of dual therapy has been shown mainly by studies in which lower doses of HCQ were used (400 mg / day, for a total maximum of 4400 mg throughout treatment).
A partial analysis of only 4 randomized studies (RCTs), in which high doses of the drug were used, showed no significant therapeutic effects. It should be noted that the RCT studies include the two large WHO trials, RECOVERY and SOLIDARITY, which used 800 mg / d for 9 or 10 days (after the first), respectively, and a total dose of 9200 or 10,000 mg HCQ (including the first day dose), respectively. A very high dose of treatment compared to the rest of the studies, in particular the observational studies.
The plausible hypothesis developed by the authors of the meta-analysis is that the result, which is different from that of observational studies, is due to the fact that randomization makes it possible to show that the effectiveness of the treatment is strongly dependent on its dosage, with optimal doses well below those given in randomized trials. On the other hand, national guidelines suggested (before the drug was banned) to use HCQ 200 mg twice a day for 5-7 days; probably to maintain a better risk-benefit profile.
However, with regards to the safety of the drug, it should be noted that despite the high prevalence of cardiovascular disease in patients with COVID-19 or the high dose used in CCF, no risk of cardiovascular lethality related to the disease. use of HCQ has not been observed. In addition to the sample size, other strengths of this meta-analysis are as follows:
- inclusion of all recently published data not included in previous meta-analyzes;
- analysis of the change in effect depending on the dosage of hydroxychloroquine. This is the broadest and most comprehensive quantitative overview of the association between HCQ and mortality in patients with COVID-19.
In addition to these studies, a living meta-analysis of 153 studies is expected to be reported which, on December 3, 2020, provides absolutely positive evidence on the use of HCQ in the early stage of COVID-19: HCQ is effective against Covid- 19 when used in early phase analysis of 153 studies . ( Covid Analysis , October 20, 2020 (Version 26, December 2, 2020).
The meta-analysis infographic is reproduced below:

Results:
- HCQ is effective for COVID-19. The likelihood that ineffective treatment will produce positive results such as those seen in the 153 studies reviewed to date is 1 in 9 billion (p = 0.00000000000011).
- Early treatment is the most effective: 100% of studies report an estimated reduction in measured endpoints (hospitalization, death, etc.) from the mean of 65%, based on a meta-analysis. random effects (therefore more conservative in estimating the effect) 95% confidence interval of the risk reduction: 0.27 to 0.46.
- In 100% of randomized controlled trials (RCTs) for early treatment, pre-exposure or post-exposure to the virus, the probability of observing the results obtained if the drug were not effective would be 0.00098.
- There is evidence of publication bias in favor of negative results (identified with special statistical tools). For example, 89% of prospective studies report positive results, while only 76% of retrospective studies do. In addition, in the United States, where the drug has a political connotation due to certain remarks by President Trump, there are many more studies with negative results than in the rest of the world, with very great importance: p = 0.0007.
1.2.12 Prodromos and Rumschlag (2020) present a systematic review of 43 studies and 32 hospitalizations where HCQ was found to be effective when administered on an outpatient basis: 11 out of 11 studies in patients at home demonstrated the efficacy, 9 of which were statistically significant , 2 were statistically favorable . Overall, in all studies (on patients, HCQ was effective in 53% of the studies reviewed and, removing studies with obvious bias, this figure rises to 75%. There were no adverse events. seriousness or mortality due to HCQ in the studies analyzed.The authors conclude:
We do not believe that randomized controlled studies are necessary before HCQ is cleared for systemic use because the efficacy seen in studies previously performed indicates that patients under control in such studies may die unnecessarily , and because that the delay in carrying out such a study would cause even more deaths by preventing the use of HCQ when it is most useful: that is, immediately!
Our study has shown that there is good evidence for efficacy and that there are no risk, cost, or supply factors for not treating now. The risk of unnecessary death of a patient caused by a delay in the use of the treatment is too high a price to pay compared to the advantage given by a greater certainty of knowledge. Many of them may have already died needlessly as a result of inaccurate information about HCQ.
It is imperative that we do not further increase the number of COVID-19 deaths by refusing to prescribe HCQ.
1.2.13 CORIST Study . retrospective Italian observational study, in which they were analyzed 3,451 unselected patients admitted to 33 clinical centers in Italy (including Spallanzani, Gemelli, Humanitas), from February 19, 2020 to May 23, 2020. The use of HCQ in hospitalized patients with COVID-19 has been associated with a 30% lower risk of death, particularly in patients with an elevated PCR test.
In total, 539 Covid-19 hospitalized patients were included in the Milan cohort, from February 24 to May 17, 2020. After adjustment for several key confounding factors (age, sex, number of concussions, cardiovascular disease, date of death) hospitalization, basal plasma), the use of hydroxychloroquine + azithromycin was associated with a 66% reduction in the risk of death compared to controls.
In addition, the study shows a strong interaction (p-value 0.0001) between efficacy and improvement of the pathology, confirming the hypothesis of the efficacy of the drug at an early stage . All “confounding” factors were excluded from the analysis.
The study is based on real life in Italy and this makes it even more interesting for regulatory purposes. Although CORIST is clearly positive, AIFA argues that the deaths could be linked to the toxicity of HCQ, which is highly unlikely given the dosages and results of previously published studies.
1.3 Binomial tests on observational HCQ data at the start of therapy
The case series do not have a control arm, but the results obtained at the chosen endpoint, the hospitalization rate, can be compared to those of the general population subjected to the standard of care before the introduction of protocols. treatments based on HCQ (or other drugs such as ivermectin, remdesivir, etc.). This rate was around 20.4% just before the introduction of these protocols, according to data from the ISS (Higher Institute of Health). Covid-19 Outbreak Report National Update March 26, 2020 – 4 p.m.
We used this estimate as a benchmark, because it’s official and because it’s the most conservative around (using the others we would have even more meaningful results).
The other estimates are around 24.9% of the Integrated COVID-19 Surveillance Bulletin from March 19 to 26, 2020 (20.4% with severe symptoms requiring hospitalization + 4.5% with a critical clinical picture requiring hospitalization in intensive care) and 37, 38% from data from official bulletins of the Italian Civil Protection from February 24, 2020 to March 23, 2020 ( data source ).
Assuming a zero effect of HCQ in terms of reducing the hospitalization rate, we performed a binomial test on each case series to see if the relative hospitalization rate differed statistically significantly from that of the general untreated population. The probability expressed in the last column of Table 2 expresses the probability of observing these results if the treatment had no effect. This probability is extremely low in almost all studies.

Null hypothesis H0: The probability of hospitalization of patients treated with HCQ is not less than that of the general population subjected to SoC.
Alternative HA hypothesis: the probability of hospitalization of patients treated with HCQ is lower than that of the general population subjected to SoC.
* Hospitalization rate in the general population treated with SoC (Standard of Care).
For each series of cases, the test result is very or extremely significant, with the exception of Montagnani (2020), who nevertheless shows a favorable trend. It should be noted that none of the studies exhibited cardiotoxicity or other side effects (if not minor, such as gastrointestinal disturbances) and no deaths (excluding 5 of 1061 patients in the study. Million et al. 2020 and 2 out of 405 patients in the Zelenko et al. 2020 study). However, the deaths are due to Covid-19 not to HCQ.
1.2 Studies cited by AIFA
Here are the 22 studies used by the IAAF to support the decision to ban the use of hydroxychloroquine outside of randomized trials, highlighting their methodological limitations and their uselessness with regard to the external validity criterion.
1.2.1 The RECOVERY trial is conducted on hospitalized patients and measures the mortality rate and length of hospital stay. The loading dose is 2000 mg, which is double the maximum dose of the therapeutic dose recommended in the literature (similar considerations apply to Solidarity, see below). In this trial, the death rate in both groups – HCQ and control, is very high and inexplicable: 25.7% and 23.5% respectively. However, the patients in the study are hospitalized and at an advanced stage, so the results are not directly the subject of our investigation (HCQ administered at an early stage).
1.2.2 Skipper et al. 2020: This is a publication of the same Boulware study (see below) which therefore inherits all the methodological imperfections in terms of
- measurement of the effect: on inclusion, patients have symptoms compatible with those of COVID-19 and are subject to a high risk of exposure or are Covid patients confirmed with laboratory tests ( only 58% ); after treatment, subjective measures of perceived severity of symptoms;
- study design (recruitment of symptomatic subjects via the Internet, without medical support in the treatment),
- data analysis: division into subgroups so as not to obtain statistically significant effects with the measure of the effect which is the absolute difference in risk rather than relative (see below).
Although the endpoints used show no statistically significant difference, there were 10 hospitalizations in the placebo group, including one death, while in the HCQ group, hospitalizations were less than half (4) and one death ( not hospitalized). The small number does not allow a statistically significant effect, but the trend is fairly indicative (p = 0.29). Boulware is the corresponding author of this study and the next, of which he is the first author. It is clear from his public biography and the declarations of interest at the bottom of the articles that Boulware has obvious conflicts of interest, since he has a structured collaborative relationship with Gilead., manufacturer of the drug Remdesivir, of which HCQ is undoubtedly a potential competitor. The conflicts of interest section of this article also declares anonymous private lenders.
1.2.3 Boulware et al. 2020: Boulware’s study was also analyzed by Watanabe (2020), who criticizes his research design , the measurement of effects and the choice of statistical test. The research project involved the recruitment via the Internet of asymptomatic subjects who previously declared themselves exposed to the virus through contact lasting more than 10 min and a distance of less than 0.6 m with people positive for the virus. Participants were then randomized and sent them a package with hydroxychloroquine (strength 800, then 600 mg) or placebo and instructions for do-it-yourself at home (with a fairly high dosage compared to protocols used by general practitioners).
The measure of the effect was defined for people presenting symptoms compatible with COVID, no diagnostic test was planned at the start or at the end of the study. This irreparably invalidates Fisher’s statistical test because the proportion of infected (symptomatic and asymptomatic) cannot be determined with precision.
This flaw in the research design could have been resolved in the analysis of the data by adopting as the measure of the effect the relative difference in risk rather than the absolute difference. The relative difference is calculated using the following formula:
Dr = (% symptomatic HCQ group -% symptomatic placebo group) / (% symptomatic placebo group
Dr = (# symptomatic placebo group / N) – (# symptomatic placebo group / N)) / (# symptomatic placebo group / N)
Since N is present in both the numerator and denominator, it becomes simpler and the relative measurement is unaffected by sample size.
Instead, the formula for the absolute difference measure is:
Da = (# symptomatic HCQ group – # symptomatic placebo group) / N
If we added to the sample a number of M uninfected patients in each group (HCQ and control) Dr would not be affected, while Da would be.
In fact, using Dr on the same data provided by Boulware et al. (instead of Da as analyzed by them), the HCQ-treated group appeared to have a 12% reduced risk rate for symptoms in the HCQ group.
In addition, the use of the Fisher test for the subgroups identified post hoc is criticized, and not for the entire sample established by protocol (treatment with HCQ from 1 to 3 days from the moment of exposure alleged); using this group, the result would have been statistically significant (as also confirmed by the France Soir 2020 collective) . Secondary analysis (Yang et al. In 2020) confirms this result. By reanalyzing the data, the authors note their statistical significance (p-value 0.0496).
In addition to the various secondary analyzes, Boulware’s study has been severely criticized in the magazine’s comment section (to date 11 comments) and in letters to the editor (to date three). So much so that the editor of the New England Journal of Medicine, while not withdrawing the study, was forced to publish an editorial on the subject, identifying its various methodological flaws, particularly with regard to the research plan (Cohen 2020).
1.2.4 Cavalcanti et al. This is a study conducted on hospitalized subjects, 42% of whom are already on oxygen therapy at the baseline. Not relevant for our purposes .
1.2.5 Abd-Elsalam et al. 194 patients in three subgroups, this stratification does not provide statistical significance. Of the three subgroups, the one potentially relevant to patients of mild severity, is extremely small in sample size: 23 patients assigned to the HCQ arm and 39 to the control.
1.2.6 TEACH STUDY: randomized study of 128 hospitalized patients, who, it is reiterated, are not the target that we propose. We note that the absence of a difference in efficacy between HCQ and placebo also concerns the side effects, for which the stage of the pathology does not play any role (if not exacerbating). This study is therefore relevant for the evaluation of the safety of the drug in positive terms.
1.2.7 Abella et al. 2020: This study recruited a small number of subjects (64 in the HCQ group and 61 in the control group). This is an early phase randomized trial. He studied the prophylactic effect, an effect that is neither directly related nor central to our analysis, but uses another rationale: hydroxychloroquine works in the early phase for multiple effects: antiviral, immunomodulator and, and antithrombotic. The prophylactic effect was not studied.
1.2.8 Solidarity trial : Study of 11,266 randomized patients whose intra-hospital mortality was analyzed. The study provides for a loading dose of 2000 mg . In the publication, this dose is justified by reference to the amoebic abscess of the liver, but in the literature for this pathology the toxic lethal dose which presents a risk of death is 1,000 mg. Thus, the study dosage is exactly double the maximum predicted in the literature. In addition, the study setting is inadequate to establish the therapeutic properties of HCQ in the early stage of COVID-19 pathology. Finally, as the study compares various treatment options beyond HCQ, such as ivermectin and Remdesivir,it is difficult to follow the logic of the decision to continue authorizing the prescription of Remdesivir , which in the study did not obtain more favorable results than HCQ and at the same time to ban the use of HCQ.
1.2.9 Rajansingham et al.This is one of the three publications to emerge from the same study by Boulware et al. This analyzes data on 1,483 healthcare professionals at risk from Covid19 aerosols. Unfortunately, by the authors’ own admission, the study cannot reach statistical significance, because the sample does not reach the predetermined number necessary to be able to observe the therapeutic effect at a contagion rate estimated at 10% ( n = 3150). However, the authors analyze data resulting from sample n = 2271, which may not necessarily achieve a significant effect, regardless of the actual effect of HCQ. There are 29 subjects infected with Covid (confirmed by PCR tests or on the basis of “COVID-compatible symptoms) in the HCQ group with a dose once a week, 29 in the HCQ group with bi-weekly dosing and 39 in the placebo group. The figures are undersized to pass a two-tailed test with α = 0.025 (for both regimens), which the authors set out to do.
It should be noted, however, that the measure of effect is very biased, as in Skipper et al. and Boulware et al., the other publications resulting from the same study (protocol, design of research and recruitment subjects). Rajansingham, Skipper and Boulware, therefore, should be viewed not as separate and independent studies, but as different publications emerging from the same study .
1.2.10 Self et al. Randomized study conducted in 34 hospitals on patients with COVID-19 in severe condition, of which 20.1% in intensive care; 46.8% supplemental oxygen; 11.5% in non-invasive ventilation or high flow oxygenation; 6.7% in invasive ventilation or extracorporeal oxygenation. Although this is a methodologically flawless study, it is not relevant for the identification of the therapeutic effects of hydroxychloroquine in early treatment.
1.2.11 Mitja et al.This is a randomized study carried out in Catalonia, on 293 non-hospitalized patients, treated within 5 days of the onset of (mild) symptoms with hydroxychloroquine (800 mg on the first day then 400 mg per day for 6 days) or placebo, which assesses reduction of viral RNA in nasopharyngeal swab as the primary outcome and worsening / resolution of symptoms and adverse events at 28 days as secondary outcomes. 80% of patients are healthcare professionals. The results indicate that patients treated with hydroxychloroquine do not have a lower risk of hospitalization and reduced healing time than the group treated with placebo, but also underline the absence of significant adverse events related to the treatment (defined as the worsening of the symptom already present or the appearance of new symptoms). The case studies treated with hydroxychloroquine are of 136 patients; the mean age is relatively low (mean 41.6 years) and the time between positivity of the first swab with which patients are reported for the study and the baseline is not specified, but only that the first visit takes place within 5 days of onset of symptoms. All of these and the fact that the suitability of patients for enrollment in the study is determined by mild symptoms, the resolution of which can occur regardless of the use of hydroxychloroquine or placebo, may suggest that the absence of a significant difference between the two groups is rather due to these elements. In fact,the confidence interval for the difference in hospitalization rate in the two groups, measured in terms of risk ratio (RR) is quite wide (95% CI, 0.32 to 1.7).
1.2.12 Lane et al. To assess the safety of hydroxychloroquine alone or with azithromycin in patients with rheumatoid arthritis in short and long term therapy. The comparison is not with the placebo, but with another drug, sulfazalasin, generally prescribed for the same type of therapeutic indication. For the comparison of HCQ + Azithromycin combination therapy, the alternative antibiotic chosen is amoxicillin.
In the short term (1-30 days), which concerns us here, HCQ is safe, while in the long term, not relevant to our treatment protocol, the study detects cardio-toxicological issues (no death) and QT prolongation. In combination with azithromycin, HCQ therapy is less safe than AZ therapy. Unfortunately, the link to the additional data that would allow the data analysis to be examined is not responding; we therefore limit ourselves to an evaluation of the study based on the main article.
The construction of the works is very open to criticism. The data comes retrospectively from databases in different countries that have very different health facilities. The use of chronic hydroxychloroquine in these patients is understandable; however, to justify the addition of azithromycin, we must refer to situations which are believed to be acute superinfections. Thus, in this data collection, we mix the chronic and acute cases linked to superinfections, on the other hand not to report exactly the dosage of the different drugs.
Judging from Figure 1, it would appear that in the group with hydroxychloroquine there are more patients with advanced renal failure and therefore more prone to cardiovascular mortality and stroke. This makes the comparison between patients treated with hydroxychloroquine therapy and the control group unbalanced.
1.2.13. J Magagnoli et al. US Veterans Hospital Electronic Database Retrospective Study. Patients who entered hospital within 24 hours of being diagnosed with COVID-19.
However, it is not known how long they had symptoms and how they were treated before entering the hospital. According to the tables describing the clinical characteristics of the patients, there is already a rather deteriorated state of health in the three comparison groups (HCQ; HCQ + AZ, no HCQ): The criteria of interest for the study are ventilation and death.
From the first table, it turns out that the patients left in the hospital (so it is assumed to be more severe) are around 18% with HCQ, 12% with HCQ + AZ, and almost 30% in controls. Extending follow-up would most likely have reversed the data compared to the control group. The study was funded not only by public research organizations, but also under the IMI initiative, a research fund co-funded by the European Federation of Pharmaceutical Industries. In the section on conflicts of interest, the authors report systematic collaborations with many pharmaceutical companies. This applies to many of the studies opposed to the HCQ.
1.2.14 Paccoud et al .: this is a retrospective study, on an insignificant number of patients; the HCQ group includes around thirty people. There is no basis to assess the effects for or against the drug.
1.2.15 Yu et al .:retrospective study of 550 critical patients, hospitalized in Wuhan between February 1 and April 4, 2020, treated with HCQ at a dose of 400 mg / day. The main end point is the difference in the death rate of the HCQ vs. control group, the secondary, the reduction in il6 levels as an indicator of the anti-inflammatory action of the drug. The results are extremely positive for both evaluation criteria. The mortality rate in the group is 18.8% (9 out of 48 patients), while that of the control group is 47.4% (238 out of 502 patients) with a high level of statistical significance (p <0.001) . The level of Il6 significantly decreases in the HCQ group, for the subgroup with a level> 60 pg mL, this level decreases with the duration of administration while it increases in the control group. The authors further test the relationship between HCQ and IL6 by checking whether the level of IL6 increased upon discontinuation of administration. This test was also found to be successful, as Il6 levels consistently increased in patients who stopped taking HCQ.
The authors check for differences that might exist in the baseline covariates and do not detect any, except for a different proportion of interferon intake in the two groups (p 0.01), so that it is not unclear why AIFA considered this poorly interpretable study due to unspecified biases.
1.2.16 Arshad et al .: retrospective analysis of 550 cases hospitalized for severe respiratory failure, where the disease has already caused some damage to the respiratory system, to the extent that respiratory support was necessary; it is therefore a type of patient who does not belong in any way to the home care establishment. The combination of hydroxychloroquine + azithromycin has been reserved for selected patients with COVID-19).
The Kaplan-Meier curves, however, show a higher survival rate in the groups of patients treated with HCQ or HCQ + Azithromycin, compared to the groups not treated with HCQ (or only with azithromycin). The curves also show that the survival rate continues to be higher in the HCQ group than in other groups up to 28 days after hospital admission. The study’s conclusion highlights that early onset of therapy, even in these compromised patients, had clear benefits.
We also underline as an important fact pointed out by the authors, the fact that none of the patients had torsades de pointes. This further confirms the safety of the treatment, even in severe patients. It is therefore a positive study in favor of HCQ (both in terms of mortality reduction and safety).
1.2.16 CORIST study: Observational study of 3451 patients admitted to 33 centers in Italy. 76.3% of patients were treated with HCQ with a 30% reduction in in-hospital mortality and morbidity (hazard ratio 0.70, CI 0.59-0.84). Despite the limitations of the observational study, the authors state the need to consider HCQ as part of the treatment for COVID-19: “The use of HCQ has been associated with a 30% lower risk of death in hospitalized patients. Covid-19. Within the limits of an observational study and pending the results of randomized controlled trials, these data do not discourage the use of HCQ in hospitalized patients with Covid-19.
1.2.16 Catteau et al.Observational study with a very large sample of hospitalized subjects: 8075 including 4542 in HCQ monotherapy and 3533 in the control group (supportive care). Deaths were recorded in 17.7% of subjects in the treatment group versus 27.1% in the control group, this difference corresponds to an adjusted risk ratio of 0.684 (with a 95% confidence interval between 0.617 and 0.758) , which is equivalent to a difference in mortality of 30% for hospitalized patients receiving HCQ treatment. The adjusted risk ratio takes into account possible history examined, such as comorbidity, age, smoking, etc. (see figure 2). In the sample, there is also a difference between the subgroup of those who start therapy in the first 5 days from the onset of symptoms compared to those who start it in the following days; but the adjusted risk ratio remains favorable for HCQ even in the second subgroup. We reproduce below the cumulative incidence of mortality as a function of the duration of the pathology, measured by inverse propensity-weighted score, i.e. taking into account the base differences of the two groups (HCQ; control ):

Compared to the control therapy, the low dosage of HCQ in monotherapy is associated, independently of other possible co-factors, with lower mortality in hospitalized patients for COVID-19.
The AIFA report qualifies these results as difficult to interpret because the two groups (HCQ and control) were different at baseline, ie at the start of the study. But the measure used by the authors took this into account, using a weighted propensity score. So it seems that the IAAF may not have been able to interpret the methodological description of the study: “The propensity for treatment with HCQ was estimated from these same basic covariates (R package IPW v.1.0-11). A normalized inversely weighted cumulative incidence of propensity for in-hospital death for each treatment was derived using the RISCA R package v.0.8.2 [24]. Competing risk analysis was repeated for patients treated within 5 days of onset of symptoms or beyond. Sensitivity analyzes were performed (additional material): they examined additional fits in the model, possible immortal time bias associated with delayed reception of treatment.
1.2.19 Gentry CA et al .: Retrospective cohort study conducted on March 1, 2020 veterans aged 18 and over undergoing medical treatment for rheumatoid arthritis, lupus and other rheumatic conditions. The objective was to evaluate the long-term prophylactic effect of HCQ in the prevention of infection with SARS-CoV2 between 1/03/2020 and 30/06/2020.
10,703 patients were treated with HCQ against 21406. No difference in the development of the infection (0.3% vs. 0.4%). No difference in results was identified between the two groups of those who developed an active infection.
Interestingly, however, the overall mortality was lower in the HCQ-treated group (p = 0.0031). The authors do not find an explanation for the reduction in mortality. Of course, although not explicit, this study also confirms the safety of HCQ , in the long term at the dose equal to 400 mg / day.
1.2.20 Yost et al. Review based on a series of studies controversial for their high dosage (such as SOLIDARITY and RECOVERY) or irrelevant because they are performed on patients with advanced disease or prophylaxis. The review also puts individual studies, secondary analysis, and meta-analysis on par, which affects the overall rating because it has double counts (some studies are included individually and in meta-analysis). analyzes mentioned). Similar problems are found in the next study (Howard et al.).
1.2.21 Howard et al.This is a succinct analysis in which recommendations are formulated on the basis of a “BET” (Best Evidence Topic Report); analysis which favors the criterion of internal validity and therefore differs from the systematic review (a systematic sampling of all relevant studies on the subject). Due to the small number of studies performed at the time of its development, the review actually includes studies of various types, including observational and uncontrolled studies. These are studies on hospitalized patients at the advanced stage of the pathology (confirmed COVID pneumonia and need for oxygen) and / or with doses higher than the protocol that we have proposed (600mg vs 400mg). In studies with moderate dosage and symptomatology the results are all positive (Gautret et al 2020 a, b). The analysis also includes a secondary analysis of one of the studies which gave positive evidence (which refutes its statistical significance): an absolutely heterodox procedure and devoid of any methodological justification. The final recommendations are influenced by the high selectivity of the studies and the distorted analysis of the results of the included studies.
1.2.22 Axfors et al .. This is a prepublication that collects 63 studies. However, the studies which weigh abnormally in the identification of the champion treatment are Recovery and Solidarity which represent 67% of the sample. This meta-analysis emphasizes relatively high dosages, in fact much higher than the standard of treatment, in this case twice the maximum recommended for this treatment in its treatment recommendation. The expected result can therefore only be negative with regard to mortality from all causes; with an OR 1.11 (95% CI: 1.02, 1.20;).
1.5 Pharmacological considerations
Hydroxychloroquine has been a drug known for decades for the treatment of patients with lupus erythematosus, rheumatoid arthritis and malaria precisely because of its effective action to reduce the levels of anti-phospholipid antibodies (E. Nuri et al., 2017). In vivo and in vitro studies show that HCQ is active against SARS-CoV-2 by inhibiting nucleic acid synthesis, viral protein glycosylation, viral assembly, and virus release (Liu et al. 2020 , Maisonasse et al. 2020, Wang et al. 2020,). In particular, the pharmacological action of the molecule is multiple:
- increase in endosomal pH leading to inhibition of fusion between cell membrane and virus;
- inhibition of glycosylation of ACE2 cell receptors, which further interferes with the link between the virus and the ACE2 receptor;
- inhibition of virus transport from endosome to endolysome (necessary for the release of the viral genome inside the cell);
- immunomodulatory effects.
Neves et al. (2020) detects a dosage-response relationship between the use of HCQ and the severity of symptoms, which provides further evidence for the pharmacological action of the drug in combating the effects of the virus. Already in 2005 several articles published by the NIH in the journal Virology had noted the efficacy and safety of HCQ for Sars-cov-1 (Vincent et al. 2005, see also Rolain et al. In 2007).
1.6. Drug safety
HCQ has been on the market since 1959 for chronic diseases. Several studies have demonstrated the safety of the use of HCQ both in prolonged therapy and in short treatment cycles with doses not necessarily high, even 200 mg twice a day (M. Million et al. of patients with COVID-19 by hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis 2020; 35: 101738).
Risch’s (2020) review presents numerous studies to support the absolute safety of the drug, obviously when used in therapeutic doses and not given to patients susceptible to cardiotoxic reactions. Our protocol provides for a treatment duration of up to 10 days per 400 mg dose.
Heard for an opinion on the subject, Professor Roberto Gerli, president of the Italian Society of Rheumatology, confirmed, “they have been using it for 35 years and they have not recorded significant adverse effects at the cardiological level” . The drug is currently used in Italy by around 60 thousand patients suffering from rheumatoid arthritis or lupus. The drug does not give significant risks at the dosage of 400 mg. A drug that has been used for years in the therapies followed cannot have a negative risk profile for short-term therapies such as that provided for in our protocol.
QT interval extension by itself does not constitute an adverse event comparable to the fatal results of coronavirus infection if left on its own in particularly frail subjects. In addition, as the cardiac risk is considered a contraindication for HCQ, the attending physician obviously takes it into account in the treatment decision.
In addition to the decades-long safety guarantees of HCQ resulting from prolonged and continuous use for a large audience of patients with rheumatoid arthritis or lupus, and the indications of the aforementioned studies, a study specifically devoted to the examination of the association between HCQ and cardiac risks (Pascarella et al., 2020), shows that the use of HCQ is not associated with the length of the QT interval in a large cohort of patients with SLE and RA.
Safety data for HCQ were recently presented to the American College of Rheumatology Annual Meeting in an article titled Use of HCQ was not associated with increased QT interval in a large cohort. of patients with LSL and RA , ParkE, et al. 2020 (study of 681 patients: no feedback on QT prolongation).
Another report on the safety of HCQ for Covid 19 is published in the journal New Microbe and New Infect 2020; 37: 100747) https://doi.org/10.1016/j.nmni.2020.100747 Hydroxychloroquine is protective for the heart, not harmful.
This is a review of studies available on major search engines that included both case reports, prospective co-reports, and retrospective reports. With the exception of some reports of non-serious effects (QT prolongation in 23% of subjects), HCQ is even associated with a reduced incidence of fatal adverse cardiac events.
In particular, no cases of death related to the main twist, which had rather impressive importance in the media. The combination with azithromycin is also safe. The figure may come as a surprise, but it actually coincides with other observations about the cardiologic safety of HCQ.
According to the review of the literature, the drug reduces thrombosis, especially the coronary artery and arithmetic. Interesting is the reference to the work of Hung 2018 describing a reduced risk of coronary heart disease in patients with rheumatoid arthritis treated with HCQ. According to data from Sharma in 2016 (cited in the review), patients with rheumatoid arthritis treated with HCQ had a 72% reduction in cardiovascular risk. These observations would explain the reduction in overall mortality in the GENTRY et al. 2020, which analyzed hcq in veterans with rheumatic diseases demonstrating increased survival over controls, on the same basis as other immunomodulatory drugs and steroids.
HCQ, the authors conclude, should not be prevented from COVID 19 patients for fear of fatal heart events that are not proven.
Finally, the authors even go so far as to propose HCQ as a potentially protective drug to treat COVID-related heart disease.
Psychiatric symptoms
A separate chapter is devoted to alarms about the risk of depression and psychotic disorders in patients treated with HCQ. It was recently launched by the EMA through its Safety Committee, and reports 198 since the launch of a literature review in May 2020, based on a report published by the Spanish Agency drugs in June 2020 (AEMPS (2020). However, it appears that the agency in turn relied on a total of 6 cases treated at higher doses at least according to what the EMA mentioned.
The Spanish Agency’s website does not report bibliographic citations regarding the reported cases and the only citations refer either to psychiatric disorders caused by the pandemic or to previous work in Lupus (which, among other things, -even associated with psychiatric pathology).
Communication of this alert has spread in unusual and inappropriate ways through news agencies , even to the scientific community.
This information has aroused amazement and disbelief in patients who have been in treatment for years, while causing an alert in the community and potential new patients, or in those who have been in care in recent times ( nocebo effect).
It should also be noted that the risk of depression with suicidal ideation is associated as a rare event in many commonly used drugs from certain antibiotics, switching to antidepressants, antiepileptics, etc. without for this reason the therapeutic use of such is considerably limited as is the case with HCQ.
In a comprehensive review of the literature on the correlation between chloroquine and HCQ and psychiatric disorders, published in DRUG SAFETY by a French group on 19.10.2020 (García et al. 2020), the psychiatric events detected worldwide over 1754 Reports of HCQ use are 56, of which only 4 suicides, 3 cases of self-harm, 12 cases of hallucination.
These cases are not found in the bibliography; while some bibliographic entries in the study date back to the 1960s and are applied directly by the authors to the use of HCQ in COVID-19 therapy, without reference to any published article specifically on the subject.
On the other hand, the authors cite extensive documentary data that points to the risk of psycho-social distress, including suicide, resulting from the COVID-19 pandemic: uncertainty, anxiety, social isolation, economic problems, as causes of unrest. psychiatric – factors well described also in a work by Gunnel published in Lancet Psychiatry in April 2020 and also taken up by Jama Psychiatry.
The caution in the treatment of psychiatric patients, as recommended by the EMA, is part of the good practice of patient clinical supervision, before prescribing any therapy (eg aspirin in patients with a history of ulcer).
2. Decision making
If one excludes the hotly debated Remdesivir (of which the IAAF recently reduced the use), and other drugs such as heparin and cortisone (also excluded for recent ministerial guidelines), and antibiotics (when the virus is active), which form the backbone of the combination therapy that we offer, there is currently no valid alternative to the use of HCQ, especially in the early stages pathology and in the family environment.
The therapy decreases the aggravation of the pathology , so that it has a very high clinical value in terms of not only healing, but drastically reducing any irreversible damage that might also occur in those who recover from the pathology, having suffered the most dramatic effects.
The fact that hydroxychloroquine prevents the worsening of the disease, and therefore hospitalization, also offers enormous advantages from the point of view of maintaining the health system and public expenditure: it is estimated at around 100 euros of care totals with HCQ against 700-2500 euros per day in the hospital, depending on the inpatient care framework. It should also not be forgotten that hospitals are known to be a very significant hotbed of the pandemic. Reducing hospitalization would therefore mean reducing contagion, precisely in the places where it is most dangerous and becomes fatal.
In addition, reduce the hospitalization of at least part of COVID patients, free beds for non-COVID patients, suffering from diseases with high care and commitment of specialists (cancer, heart disease, surgery, etc.) to give a few examples, in the first six months of 2020, 1,400,000 fewer cancer screenings were taken than the previous year and there is an increase in colon cancer mortality of 11.9% ( and 60% for cardiovascular disease).
Also, it is a short-term therapy; which reduces the likelihood of side effects and their possible severity. HCQ is a proven drug, used for decades for rheumatoid arthritis and lupus erythematosus, without any particular heart risks.
It is incomprehensible to see how hydroxychloroquine can have a positive benefit for rheumatoid arthritis (chronic condition, but not lethal, which requires continued use of the drug) and a negative effect with regard to COVID-19, which leads to deterioration of clinical health rapidly and can be fatal, and for which early treatment with hydroxychloroquine is limited in time.
The AIFA’s decision to ban the use of HCQ outside of clinical trials is therefore in strong opposition to many ethical principles of the medical profession and decision-making rules in the field of uncertainty, such as the precautionary principle. .
Faced with the absence of any valid alternative, and the severity of a pathology, especially if the only alternative is to allow the disease to progress in the weakest subjects, it is normal to give authorization for the use of a drug that is over the counter and expanding its use for the treatment of COVID
Regardless of the supposed harmful effects of HCQ, these will always be less than the lethal result of contracting the virus in particularly debilitated subjects.
The AIFA approved the use of HCQ on the basis of favorable data in the Official Journal of March 17, granting its reimbursement for home care (ADI COVID), and protecting the liability of doctors.
However, the AIFA did not sufficiently take into account all the territorial experiences collecting useful data to assess the therapeutic value of HCQ at a time when Italy was in the eye of the storm. After the decision published in the Official Journal of March 17, 2020, the monitoring tools were put in place: the legal directive ruled on the use of HCQ in an integrated home care system, with monitoring of health districts . It would have been enough to ask the ASL to make a report to have a real case study on thousands of cases.
The AIFA withdrew its authorization after less than 2 months, on the sole basis of the literature (study Mehra et al., 2020 published in the Lancet and then withdrawn), without resorting to the knowledge acquired by physicians engaged in the field. , such as General Practitioners from National Health Services.
They could have answered the key question: is HCQ effective in reducing the rate of hospitalization? This makes the restriction on the prescription of the drug even more incomprehensible.
It is also very disconcerting that after the safety alert which led to the suspension of the use of HCA, no verification of the impact of the treatment with HCQ on the level of the Italian population has been undertaken.
The table below shows the drug consumption between the pre-Covid-19 period, identified during the December 2019-February 2020 quarter, and the following ones, from March to May 2020 (IAAF report on drug consumption July 2020).

HCQ consumption increased by + 4661%.
Why did the AIFA not conduct a toxicity study on treated patients, while it included studies carried out abroad in contexts other than those structured in Italy?
2.1 Clinical assessment and division of skills in the health system
The attending physician is responsible for the benefit-risk assessment of the drug for each of his patients individually. It is the doctor who, depending on the history, any diagnostic examination and clinical examination of the patient can assess, if and in what amount the patient will benefit from a certain drug. The autonomy of the choice of the doctor cannot be overwritten by benefit-risk assessments relating to the population or to non-specific reference classes.
In EBM, this principle is guaranteed by the fact that clinical judgment (once relegated to the bottom of the hierarchy), has already been reassessed for years and framed in a role of synthesizing scientific evidence and translating its implications. clinics for the patient.
Once the information provided by the various studies available has been considered, it is the doctor who, in science and in conscience, can and should decide on the best therapy for his patient. The drug agency or other health surveillance and protection bodies, although it is called upon to help the doctor in the choice of treatment, by providing information and recommendations,
Although the IAAF is called upon to monitor the benefit-risk profile of the medicinal product in the general population, it cannot intervene in such an individual decision.
It is also difficult to follow the logic of AIFA’s decision to limit the use of HCQ in clinical trials.
A randomized study of HCQ is not possible under the current circumstances for clinical and organizational reasons:
2.1.1 The effectiveness of HCQ occurs within the sixth day of symptom onset, however:
- for a clinical study, it is necessary to establish the positivity of the subject by molecular swab and to obtain the result immediately given the rapid evolution of the pathology;
- in the luckiest cases, the swab takes a week to run. In some cases, it takes up to 15 days;
- the technical time for centralized randomization is one week, including the time for sending the placebo and the active drug;
It would therefore take at least 15 days to be able to put the patients in the study, that is to say three times the time in which the HCQ can act effectively. This would make the active treatment arm unnecessary.
2.1.2 The country and many hospital realities are not structured for randomized clinical trials: there are no data managers and the MPGs are overloaded with requests for IT procedures. The CRF would exacerbate increasingly complex work that is slowing difficult clinical activities in the midst of a pandemic.
2.1.3 It is also the institutional data collection systems that are lacking everywhere, but especially in the territory.
It follows that the requirement for a randomized field study is tantamount to a roundabout way of prohibiting the collection of other evidence and therefore establishing the facts.
3. Strategic plan. Research ethics, conflicts of interest and information asymmetries
The plethora of secondary reviews of published studies on the efficacy and safety of HCQ show that the stakes can create huge financial and political incentives and skew the evidence in part of the consciousness of the scientific community.
Evidence is no longer produced in the neutral context of unbiased theoretical research, but in the cited situation of a global emergency, in which huge increases in budgets for industry and research grants for scientists may be overwhelming. good motives for investigative activities and publication of results, regardless of their scientific contribution (Abbasi 2020). Scientific inquiry is therefore under pressure which can distort its processes and results. The complaint about this dynamic starts a long way and has recently manifested itself also in the so-called reproducibility crisis (Ioannidis, 2005; OSC 2015; Fang, Ferric C., R. Grant Steen, and Arturo Casadevall, 2012) .
In the BMJ, Patricia J García, MD, former Minister of Health (Peru), points out that corruption must become the object of attention and area of study by politicians, researchers, funders of the research as well as diseases. 10 to 25% of the world’s health costs have been absorbed by corruption (Garcia 2019). Experts in ethics and science policy increasingly denounce the danger to democracy posed by the politicization of science (Nature 2018, Saltelli et al. 2020; Benessia et al. 2016, Tallacchini, 2018, 2019; Jasanoff 2009; Iannuzzi 2018; Gainotti et al. In 2008).
Under these circumstances, it is plausible that the government and supranational bodies are victims of the phenomenon of “regulatory capture” . This is a phenomenon well known today in the literature which describes the effects of information asymmetry between the industry (in this case pharmaceutical) and the regulatory body, to the detriment of this the latter, which must rule on issues relevant to the industry, with information and criteria interpreting the evidence, provided by the latter.
In this asymmetrical relationship, the regulator is not only an autonomous decision-maker, since it depends on the industry as the main source of information, but ends up taking on its “state of mind” by also absorbing its axiology.
The press and media are also influenced by this kind of influence, thus helping to channel public opinion towards positions similar to those of the industry and to put pressure, in turn, on the regulator and the legislator.
Over the past few months, work has been published which directs attention to the quality of the studies themselves and to the interests that may motivate them, precisely with regard to the qeustione of the effectiveness of HCQ as a therapy. for covid-19 (see Mazhar 2020). Roussel and Raoult (2020) study the influence of conflicts of interest on public statements relating to the covid emergency, with particular attention to the Gilead case. The work measures the correlation between the funding given to university infectious diseases by the pharmaceutical company by Gilead (manufacturer of remdesivir) and their public position with regard to the potential competitor of HCQ (the correlation is statistically significant with p = 0.017).
Regulatory capture
State and Regulatory Theory and Practice perspectives show the persistence of some basic and difficult to solve fundamental problems, which, after decades of scientific reflection, still appear to be “hot topics” of the world. regulatory activity (Matteucci, 2020). Two of them are the phenomena of conflicts of interest and the consequent phenomena of capture of the regulatory body.
The analysis in this case of regulatory capture(regulatory capture, Dal Bó, 2006; Carpenter and Moss 2014) and, more generally, the capture of public authorities or institutions (OECD, 2017) have generated thousands of scientific, theoretical and empirical contributions, belonging to a wide range of academic disciplines (from law to economics, including political science, sociology, epistemology and neuroscience).
The capture of the regulator (as a public decision-maker) can occur at different intensities and, when strong (Croley, 2011), the integrity and accuracy of public decisions are compromised, as they are no longer aimed at protecting and the satisfaction of general interests (collectivity), but becomes functional to the private interests of specific groups (“rents”). There are various causes and institutional mechanisms to generate regulator capture (for a review, Mitnick, 2011).
First, there is a general state of information asymmetrywhich afflicts the regulator and the experts it uses, which stems from various phenomena: the greater experience of the regulated industry compared to the regulator (due to learning by doing, learning by doing), the difficult measurability of phenomena (especially biomedical), imperfection of the processes of experimental validation and scientific publication, and other mechanisms of deficit of public resources or institutional inertia. On this basis, the aforementioned private agendas bearing “special interests” are easy to play. These private agendas, by representing strategically (for example partially) the various risk and decision elements, can lead to erroneous or sub-optimal public decisions from the public perspective, although it is functional for private agendas.
Such conditioning power is reinforced by the fact that in some countries there is a lack of specific regulatory guarantees for transparency and regulation of lobbying activities or other stakeholder participation mechanisms (for example, that of “revolving doors”, in which regulators are then hired by the regulated, LaPira and Thomas, 2017), with which the frequency of “strong” capture may be limited.
As decades-old literature (for a recent review, Dirindin et al. 2018) shows, earlier phenomena of information asymmetry, lobbying, revolving doors and consequent regulator capture are particularly frequent and strong in the world of health, due to the economic entity of the interests at stake, the urgency in which decisions are often taken, greater economic resources belonging to private industry (which in various ways manages the of almost all clinical trials), often limited resources available to the regulator and strategic use by private industry of the hierarchy of scientific evidence and publications.
Recently, the reflection on the phenomena of conflict of interests and capture progressed to more regulatory-institutional detail, and included other stakeholders (besides the regulator), and other areas of scientific consensus formation (from private sponsorship of training events) to that of the formulation of clinical practice guidelines; Norris et al 2011).
Even in Italy, episodes of capturing the same biomedical training and professional updating have been detected, which are among the most difficult to individualize – and no less pernicious than the others. Among the solutions proposed in the literature, there is the strengthening of recipes already proposed in the past (but for the most part not applied): such as the prohibition/limitation of private sponsorship of biomedical scientific events;
Finally, it should be noted that, although in the most serious cases, there are episodes of capture by corruption, extortion or embezzlement (as, for example, in the events which resulted in the conviction No. 5756 of 2012 Supreme Court, United Civil Sections), information asymmetry and private agendas can lead the regulator to make wrong or wrong decisions even if the same ones act in good faith.
For this reason, the regulator capture theory postulates various conditions for a given regulatory process to be more resistant to capture (Levi-Faur, 2011):openness to the various stakeholders, transparency of documents and access to regulatory documents, use of international benchmarking to acquire and assess evidence to the contrary, for the purposes of additional investigations.
4. Conclusions
As a result of a current major infectious event like Covid 19, its rapid spread, the devastating impact on hospital and territorial health establishments, regulatory bodies, international research networks (Cochrane in the first place) will not produce guidelines and timely information to guide the care of large numbers of patients who become ill, as is often the case with infectious diseases that spread very quickly.
The evidence presented to support the IAAF decision of its decision is in most cases drawn from hospital care settings, or even intensive care settings, where the difficulty of obtaining relevant clinical improvements in patients. patients with non-systemic impairment is evident. more manageable. It is paradoxical that in view of these results, deemed discouraging by the AIFA, it decides to limit the use of HCQ in clinical trials, which in fact amounts to limiting its use in hospitals. On the other hand, it is quite clear that if the hospital mortality is that of the last few days (around 900 deaths per day), it is clear that the hospital “cures” are not sufficient to contain the pandemic.
Faced with more than six months of experience with this disease, several published guidelines strongly advised against (including through legal decrees) any home treatment, as was instead implemented during the first wave of the pandemic. . From the analysis of the articles presented, it is clear that patients treated late have a worse prognosis, because if measures are not taken quickly, the viral infection triggers the cytokine storm, with potentially fatal autoimmune mechanisms. . The high mortality of COVID patients in recent periods is likely linked to a lack of early treatment of the disease.
At this stage of the pandemic, we must allow the possibility of treating patients at home, by promoting clinical diagnosis, with drugs of which we have sufficient knowledge for widespread and territorial use. It should also be taken into account that in these patients, hospitalization in itself represents a risk factor for mortality regardless of the causes of hospitalization (intercurrent hospital infections, aspiration pneumonia, difficulty in following disoriented, confused patients, hypoxemic …).
In this perspective, it should also be considered that for decades the use of HCQ has been authorized and safely recommended as a prophylaxis of malaria – even for prolonged periods – in healthy people, who take it without supervision. medical; or as continuous therapy for chronic pathologies, such as rheumatoid arthritis and lupus erythematosus, and instead, it is to be avoided for a pathology which, left alone, has a fatal outcome and for treatment lasting up to ‘to 10 days can favorably modify the fate of the disease.
Rome, December 7, 2020 on the spotlight
Prof Alessandro Capucci, cardiologist, former director of the School of Specialization in Cardiovascular Diseases at the Polytechnic University of Marche and director of the cardiology clinic at Torrette Hospital in Ancona. Former director of the schools of specialized thoracic surgery and vascular surgery at the Polytechnic University of Marche.
Teacher. Luigi Cavanna,
Director of the Department of Oncology-Hematology. USL company in Piacenza.
Dr Paola Varese,
Director SC Medicine and DH oncology ASLAL Piemonte Presidio Ovada
Bibliography
- Abbasi 2020 https://www.bmj.com/content/371/bmj.m4425 : Covid-19 politicization, corruption, and suppression of science. BMJ 2020; 3 71: m 4425
- ABC Eyewitness News. New coronavirus: Doctors on Long Island embrace combination drug therapy in fight against COVID-19. New York, NY: American Broadcasting Company; 2020. https://abc7ny.com/coronavirus-treatment-long-island-news-nassau-county/6093072/ . Accessed April 14, 2020.
- Abella BS, Jolkovsky EL, Biney BT, et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure prophylaxis of SARS-CoV-2 in healthcare workers: a randomized clinical trial. JAMA Intern Med. Published online September 30, 2020. doi: 10.1001 / jamainternmed.2020.6319
- AEMPS (2020): Chloroquina / Hidroxichloroquina: precauciones y vigilancia de posibles reacciones adversas en pacientes con COVID-19. Agencia Española de Medicamentos y ProductosSanitarios. 2020. https: // www.aemps.gob.es/informa/notasinformativas/medicamentosusohumano-3/seguridad- 1/2020-seguridad-1 / chloroquina-hidroxichloroquina-precauciones-y-vigilancia-de-posibles-reacciones-adversas-en- pacientes-con-covid-19 /
- Aronson JK, M. Hauben (2006) Anecdotes that provide definitive evidence. BMJ, 333 (16): 1267-69.
- Arshad et al. (2020) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7330574/
- Axfors C, Schmitt AM, Janiaud P, and others. (2020) Mortality results with hydroxychloroquine and chloroquine in COVID-19: international collaborative meta-analysis of randomized trials. medRxiv 2020.09.16.20194571; doi: https://doi.org/10.1101/2020.09.16.20194571
- Barbosa Esper R, Souza da Silva R, Costa Oikawa FT, and others. Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed by telemedicine. 2020. https://pgibertie.files.wordpress.com/2020/04/2020.04.15-journal- manuscript-finale.pdf. Accessible on April 30, 2020
- Benessia A, Funtowicz S, Giampietro M et al. The legitimate place of science: science on point. Tempe (AZ), Consortium for Science, Policy, & Outcomes, 2016
- Boulware, DR, Pullen, MF, Bangdiwala, AS, Pastick, KA, Lofgren, SM, Okafor, EC, … & Engen, NW (2020). A randomized trial of hydroxychloroquine as a postexposure prophylaxis for Covid-19. New England Journal of Medicine. DOI: 10.1056 / NEJMoa2016638
- Capucci, Alessandro; et al. Low Rate of Hospitalization Without Severe Arrhythmias: A Prospective Investigation of 350 Early-Home Patients Treated with Hydroxychloroquine During the COVID-19 Pandemic, Journal of Cardiovascular Medicine: November 2020 – Volume 21 – Issue 11 – p 922-923 doi: 10.2459 /JCM.00000000000001061 Capucci
- Carpenter DP, Moss DA, Preventing regulatory capture: Special interest influence and how to limit it, New York: Cambridge University Press
- Cassone et al. 2020: Low-Dose Hydroxychloroquine Associated with Lower Mortality in COVID-19: A Meta-analysis of 26 Studies and 44,521 Patients https://www.medrxiv.org/content/10.1101/2020.11.01.20223958v1 .full.pdf
- Catillon M, Zeckhauser R. Unlock the data on COVID-19. Boston, MA: Mossavar- Rahmani Center for Business and Government, John F. Kennedy School of Government, Harvard University; 2020. https://www.hks.harvard.edu/centers/mrcbg/news- events / COVID_Zeckhauser . Accessed April 30, 2020 We support well-meaning RCTs already underway in many medical centers. But tens of thousands of people will die before they have their results. In the meantime, anecdotal success stories, the very personal experiences of physicians, and informal information networks serve as the basis for the widespread adoption of treatment protocols.
- Catteau L, Dauby N, Montourcy M, Bottieau E, Hautekiet J, Goetghebeur E, van Ierssel S, Duysburgh E, Van Oyen H, Wyndham-Thomas C, Van Beckhoven D; Belgian Collaborative Group on Hospital Surveillance COVID-19 (2020) Low-dose hydroxychloroquine treatment and mortality in hospitalized patients with COVID-19: a national observational study involving 8,075 participants. Antimicrobial Agents Int J. Oct; 56 (4): 106144. doi: 10.1016 / j.ijantimicag.2020.106144. Epub 2020 Aug 24. PMID: 32853673; PMCID: PMC7444610.
- Luigi Cavanna, Chiara Citterio, Ilaria Toscani, Cosimo Franco, Andrea Magnacavallo, Serena Caprioli, Evelina Cattadori, Camilla Di Nunzio, Roberto Pane, Roberta Schiavo, Claudia Biasini and Massimo Ambroggi (2020). Cancer patients with COVID-19: a retrospective study of 51 patients in the district of Piacenza, Northern Italy, Sciences of the future: https://doi.org/10.2144/fsoa-2020-0157
- CEBM (2020) Guidelines from the Center for Evidence Based Medicine, Oxford: https: // www.cebm.ox.ac.uk/resources/ebm-tools/making-a- decision #: ~: text = External% 20validity % 20refers% 20to% 20the, on% 20what% 20the% 20investi gators% 20did.
- Cohen MS. (2020) Editorial – Hydroxychloroquine for the Prevention of Covid-19 – Searching for Evidence. N Engl J Med (2020). DOI: 10.1056 / NEJMe2020388.
- Le Collectif Citoyen France Soir (2020) The devil is REALLY in the detail, after The Lancet, we question the New England Journal of Medicine . France Soir, June 4, 2020: http://www.francesoir.fr/societe-sante/le-diable-est-vraiment-dans-le-detail-apres-lancet-nous-remettons-en-cause-le-nouveau
- CORIST study https://www.ejinme.com/article/S0953-6205(20)30335-6/fulltex t
Croley SP, (2011), Beyond Capture: Towards a New Theory of Regulation, in: Levi-Faur D., (2011), Handbook on the Politics of Regulation, E. Elgar
- Dal Bó, E. (2006), Regulatory Capture: A Review, Oxford Review of Economic Policy, vol. 22, n. 2, pp. 203-225.
- D’Arminio Monforte A, Tavelli A, Bai F, Marchetti G, Cozzi-Lepri A. Efficacy of hydroxychloroquine in COVID-19 disease: A deal done and dusted off ?. Int J Infect Dis. 2020; 99: 75-76. doi: 10.1016 / j.ijid.2020.07.056
- Dirindin N, Rivoiro C., De Fiore L. (2018) Conflicts of interest and health. How industries and institutions condition doctor’s choices, Bologna: Il Mulino
- Fang, Ferric C., R. Grant Sadolescent, and Arturo Casadevall, 2012, Misconduct Accounts for the Majority of Retracted Scientific Publications, Proceedings of the National Academy of Sciences, 109 (42): 17028-17033. doi: 10.1073 / pnas.1212247109
- Gainotti S, Moran N, Petrini C, Shickle D. Ethical Models underpining Responses to Threats to Public health: A Comparison of Approaches to Communicable Disease Control in Europe. Bioethics 2008; 22 (9): 466-76.
- Garcia (2019) Published: November27,2019DOI: https://doi.org/10.1016/S0140- 6736 (19) 32527-9 VOLUME 394, NUMBER 10214, P2119-2124, 07 DECEMBER 2019 https: // www.thelancet. com / journals / lancet / article / PIIS0140-6736 (19) 32527-9 / fulltext Corruption in global health: the open secret
- Garcia, P., Revet, A., Yrondi, A. et al. Psychiatric disorders and hydroxychloroquine for coronavirus disease 2019 (COVID-19): A VigiBase study. Drug Saf 43, 1315-1322 (2020). https://doi.org/10.1007/s40264-020-01013-3
- Gautret P, Lagier JC, Word P, and others. (2020a) Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 covid-19 patients with at least a six-day follow-up: a pilot observational study. Travel Med Infect Dis 2020; 34: 101663
- Gautret P, Lagier JC, Word P, and others. (2020b) Hydroxychloroquine and Azithromycin for Treatment of COVID-19: Results from an Open Non-Randomized Clinical Trial. Int J Antimicrob Agents 2020; 2020: 105949
- Gautret P, Lagier JC, Word P, and others. Hydroxychloroquine and Azithromycin for Treatment of COVID-19: Results from an Open-label Non-Randomized Clinical Trial [published online ahead of publication March 20, 2020]. Antimicrobial Agents Int J. (doi: 10.1016 / j.ijantimicag.2020.105949 ).
- Glasziou P, Chalmers I, Rawlins M, McCulloch P. When Are Randomized Trials Useless? Pick up the noise signal. BMJ 2007; 334: 349-51
- Gunnel published in Lancet psychiatry in April 2020 and also reprinted by Jama Psichiatry (JAMA Psychiatry. 2020; 77 (11): 1093-1094. Doi: 10.1001 / jamapsychiatry.2020.1060).
- Hauben M , Aronson JK (2007) Gold standards in pharmacovigilance: the use of definitive anecdotal reports of adverse drug reactions as pure gold and high-grade hours. Saf drug. 2007; 30 (8): 645-55.
- Howard L, Baombe J, Reynard CBET 2: Hydroxychloroquine in the treatment of COVID-19 Emergency Medicine Journal 2020; 37: 451-453.
- Huang M, Tang T, Pang P, and others. Treatment of COVID-19 with chloroquine. J Mol Cell Biol. 2020; 12 (4): 322-325. doi: 10.1093 / jmcb / mjaa014
- Iannuzzi A. Compulsory vaccinations in the opinion of the Constitutional Court between respecting the discretionary power of the state legislator and medical and statistical evaluations. See online 2018; I: 87-96
- Internation Journal of Infectious Diseases 2020 – Use of hydroxychloroquine and chloroquine in COVID-19: How good are the randomized controlled trials? doi: https://doi.org/10.1016/j.ijid.2020.09.1470
- Ioannidis, John P. A., 2005, Why Most Publishe Research Findings Are False, PLoS Medicine, 2 (8): e124. doi: 10.1371 / journal.pmed.0020124
- Jasanoff S. The essential parallel between science and democracy. Seed Magazine, February 17, 2009, http: //seedmagazine.com/content/article/the_essential_parallel_between_s … (last accessed: January 6, 2019)
- Journal of Cardiovascular Medicine https://journals.lww.com/jcardiovascularmedicine/Fulltext/2020/11000/Low_hospitalization_ rate_without_severe.15.aspx
- Ladapo et al. 2020:, JA, McKinnon, JE, McCullough, PA, and Risch, H. (2020). Randomized controlled trials of early ambulatory hydroxychloroquine in the prevention of infection, hospitalization, and death from COVID-19: meta-analysis. https://www.medrxiv.org/content/10.1101/2020.09.30.20204693v1
- LaPira T., Thomas HF, (2017), Revolving Door Lobbying: Public Service, Private Influence, and the Unequal Representation of Interests, Lawrence: University Press of Kansas
- Levi-Faur D., (Ed.) (2011), Manual on Regulatory Policy, E. Elgar
- Liu J, Cao R, Xu M, and others. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting infection with SARS-CoV-2 in vitro. Cell Discov. 2020; 06: 16
- AA lover. Quantifying the effects of hydroxychloroquine and azithromycin treatment for COVID-19: a secondary analysis from a non-randomized open label clinical trial (Gautret et al., 2020). MedRXiv. 2020. (doi: 10.1101 / 2020.03.22.20040949 ). Accessed April 10, 2020.
- Luco, J. (2020). Hydroxychloroquine as post-exposure prophylaxis for Covid-19: Why simple data analysis can lead to the wrong conclusions of well-designed studies
- Maisonnasse P, Guedj J, Contreras V, and others. Use of hydroxychloroquine against SARS-CoV-2 infection in non-human primates. Nature. 2020; 585 (7826): 584-587
- Matteucci, N. (2020), The State and Prospects of Regulation: A Long Term Perspective on Italy and Beyond, L’Industria, n. 3, p. 479-508
- McKee M, Britton A, Black N, McPherson K, Sanderson C, Bain C Methods in health services research. Interpretation of evidence: choice between randomized and non-randomized studies BMJ 1999; 319 (7205): 312-315
- Mehra, MR, Desai, SS, Kuy, S., Henry, TD, and Patel, AN (2020b). Cardiovascular disease, drug therapy, and mortality in COVID-19. New England Journal of Medicine. DOI : 10.1056 / NEJMoa2007621
- Mehra, MR, Desai, SS, Ruschitzka, F., And Patel, AN (2020). Hydroxychloroquine or Chloroquine with or without Macrolide for the Treatment of COVID-19: A Multinational Registry Review. The Lancet. https://doi.org/10.1016/S0140-6736(20)31180-6
- Million M, Lagier JC, Gautret P, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1,061 cases in Marseille, France [published online ahead of publication on May 5, 2020]. Travel Med Infect Dis. (doi: 10.1016 / j.tmaid.2020.101738 )
- Mitnick, B., (2011), “Capturing ̳Capture”: Definition and Mechanisms, in: Levi-Faur D., (2011), Handbook on the Politics of Regulation, E. Elgar
- Montori VM, Jaeschke R, Schunemann HJ, Bhandari M, Brozek JL, Devereaux PJ et al Users’ guide to detecting misleading claims in BMJ 2004 clinical research reports ; 329 (7474): 1093-1096
- Nature 2018. Editorial: Laws aren’t the only way to boost immunization. 553: 249-50.doi: https://doi.org/10.1038/d41586-018-00660-y
- Fabricio S Neves, Dose-response effects of hydroxychloroquine on prophylaxis against SARS-CoV-2 / COVID-19 infection, Clinical Infectious Diseases,, ciaa1807, https://doi.org/10.1093/cid/ciaa1807
- Norris SL, Holmer HK, Ogden LA, Burda BU (2011), Conflict of interest in clinical practice guideline development: a systematic review, PLoS One. ; 6 (10): e25153
- Nuri et al. (2017) Long-Term Use of Hydroxychloroquine Redices Antiphospholipid Antibodies in Levels with Primary Antiphospholipid Syndrome, Immunologic Research 2017; 65: 17-24)
- OECD (2017), Preventing Policy Capture: Integrity in Public Decision Making, OECDPublicGovernanceReviews, OECDPublishing, Paris, https://doi.org/10.1787/9789264065239-en
- Open Science Collaboration (OSC), 2015, Estimating the Reproducibility of Psychological Science, Science, 349 (6251): 943-951. doi: 10.1126 / science.aac4716
- Pascarella and a l. https://doi.org/10.1111/joim.13091 I ntern Med. 2020 May 13: 10.1111 / joim.13091. doi: 10.1111 / joim.13091 [Epub ahead of print]: COVID ‐ 19 diagnosis and management: a comprehensive review review review
- Prodromos and Rumschlag (2020) Hydroxychloroquine is effective, and consistently when provided, forCOVID-19: asystematic review https: // www.sciencedirect.com/science/article/pii/S2052297520301281 https://doi.org/10.1016 /j.nmni.2020.100776
- Rajansingham: Hydroxychloroquine as a pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial doi: https://doi.org/10.1101/2020.09.18.20197327
- Rivoiro C., De Fiore L. Dirindin N, (2019), Scientific Society and Conflict of Interest Government, Recent Advances in Medicine, vol. 110, n.3, pp. 113-114
- Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to cope with bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 2007; 30: 297-308. https://doi.org/10.1016/j.ijantimicag.2007.05.015
- Rothwell PM External validity of randomized controlled trials: to whom do the results of this trial apply ? Lancet 2005; 365 (9453): 82-93
- Savarino (2020) European Journal of Drug Metabolism and Pharmacokinetics (2020) 45: 715- 723 https://doi.org/10.1007/s13318-020-00640-6
- Schünemann H., Brożek J., Guyatt G., Oxman A. (eds) (2013) Manuel grade. Manual for ranking the quality of evidence and strength of recommendations using the GRADE approach. Chapter. 5.2.3 Indirect evidence , https://gdt.gradepro.org/app/handbook/handbook.html
- ScienceDirect – Influence of conflicts of interest on public positions during the covid-19 era, the case of Gilead Sciences https://doi.org/10.1016/j.nmni.2020.100710
- Self WH, Semler MW, Leither LM, et al. (2020) Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Jama. 2020; 324 (21): 2165-2176. doi: 10.1001 / jama.2020.22240
- Solidarity: h ttps: //www.medrxiv.org/content/10.1101/2020.10.15.20209817v 1 Antiviral drugs reused for COVID-19 – interim results of the WHO SOLIDARITY trial
- Stroppa, EM, Toscani, I., Citterio, C., Anselmi, E., Zaffignani, E., Codeluppi, M., & Cavanna, L. (2020). Coronavirus-2019 disease in cancer patients. A report of the top 25 cancer patients in a western country (Italy). Future Oncology (London, England), 16 (20), 1425-1432. https://doi.org/10.2217/fon-2020-0369
- Tallacchini MC (2019) Vaccines, science, democracy. Epidemiol Prev; 43 (1): 11-13. Doi: 10.19191 / EP19.1.P11.011
- Tallacchini M. The governance of science, from self-referentiality to systemic interactions between science, politics and democracy. Journal of Neo-Scholastic Philosophy 2018; CX, 4: 727-35
- Mohammad Tarek and A. Savarino European Journal of Drug Metabolism and Pharmacokinetics (2020) 45: 715-723 https://doi.org/10.1007/s13318-020-00640-6 :
Discussion of the pharmacological plausibility of HCQ in covid
- (Ulrich RJ et al. 2020): Treating COVID-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind Randomized Controlled Trial in Hospitalized Patients Open Forum Infectious Diseases, Volume 7, Number 10, October 2020, ofaa446 , https: / /doi.org/10.1093/ofid/ofaa446
- Vincent MJ, Bergeron E, Benjannet S, and others. Chloroquine is a potent inhibitor of the infection and spread of the SARS coronavirus. Virol J. 2005; 2:69
- Wang M, Cao R, Zhang L, and others. Remdesivir and chloroquine effectively inhibit the newly emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30 (3): 269-271
- Watanabe, M. (2020). Efficacy of hydroxychloroquine as a prophylaxis for Covid-19. arXiv preprint arXiv: 2007.09477
- Yang, AC, Liu, Y., Shao, Y., Yang, C., Xu, J., and Yang, B. (2020). Hydroxychloroquine and interferons for the prophylaxis and early treatment of covid19-current clinical advances. J Clin Cell Immunol, 11, 596. https://www.longdom.org/open-access/hydroxychloroquine-and-interferons-for-the- prophylaxis-and-early treatment-of-covid19current-clinical-advances.pdf :
- Yost, Jennifer, Itziar Etxeandia-Ikobaltzeta, Linda L. Humphrey, et al. (2020) Alert Update 2: Should Clinicians Use Chloroquine or Hydroxychloroquine Alone or in Combination with Azithromycin for the Prophylaxis or Treatment of COVID-19? American College of Physicians Living Practice Points . Ann Intern Med. 2020; 173: W88-W89. [Epub ahead of print Jul 30, 2020]. doi : 10.7326 / L20-1007
- Yu and a l. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7228868/ Yu, B., Li, C., Chen, P., Zhou, N., Wang, L., Li, J., Jiang, H., and Wang, DW (2020). The low dose of hydroxychloroquine reduces the lechality of critically ill patients with COVID-19. Science China. Life Sciences, 63 (10), 1515-1521. https://doi.org/10.1007/s11427-020-1732-2
- Zelenko V. To all healthcare professionals around the world [letter]. 2020. https://docs.google.com/document/d/1pjgHlqIZuKOziN3txQsN5zz62v3K043pR3DdhEmcos/ Accessed April 28, 2020
Note 1 According to the mathematical modeling, hydroxychloroquine can have an impact on the amplitude of the viral load peak and viral release if the drug is administered early enough (i.e. when the virus is still confined in the pharyngeal cavity). The effects of chloroquine / hydroxychloroquine can be fully explained only by also considering the ability of this drug to increase the death rate of SARS-CoV-2-infected cells, in this case by enhancing the cell-mediated immune response). (Tarek and Savarino, 2020).
Related: Piedmont gives the green light to hydroxychloroquine
Council of State: yes to the use of hydroxychloroquine for the treatment of Covid:
The AMA Quietly Admits They Lied About Hydroxychloroquine
Epidemiologist At Yale Provides Testimony On Hydroxychloroquine For Treating COVID-19
‘Only a one in 17 billion chance hydroxychloroquine doesn’t work’: medical professor

