Source: France Soir
The New England Journal of Medicine (NEJM) has just posted on February 10, 2021, a letter to the editor, entitled “ Concern about high doses of HCQ (hydroxychloroquine) in the Recovery trial ” (Concerns about high HCQ dosage in the Recovery study) in commentary to the large English randomized trial Recovery published in the same journal.
It should significantly change the dramatically distorted perception of the results of this study published in the NEJM,and lead to the re-evaluation of the benefit of HCQ as a treatment for Covid-19 in the early clinical phase and in the pauci-symptomatic phase.
Unfortunately, the Recovery trial was the only randomized controlled trial during the first phase of the epidemic to have been conducted long enough to obtain sufficient statistical power. Its negative results had led to the systematic rejection of HCQ by authorities in EU member countries, in particular France.
The letter submitted to NEJM by French researchers and clinicians at the beginning of November 2020 opens a major objection to the conclusion presented by Professors Landray and Horby, joint principal investigators of Recovery, and their collaborators.
In addition, it indicates why HCQ is active in vivo at a much lower concentration than suggested by in vitro studies . This letter has been subjected to a scrupulous peer review, just like a full scientific article. It therefore conveys an indisputable scientific significance.
Summary and simplified explanation of our objection published in the New England Journal of Medicine
The British Recovery study testing hydroxychloroquine in patients with COVID-19 used very high doses of hydroxychloroquine in its protocol, doses that we can qualify as dangerously toxic.
Hydroxychloroquine has the property of concentrating very strongly (several hundred or even thousands of times) more in lysosomes (cellular organelles where its anti-viral effect is mainly exerted) than in blood plasma.
It is therefore not necessary to give high doses of HCQ to obtain a high local concentration in the intracellular lysosomes. This property has been known for a long time.
Professor Raoult’s team from the IHU in Marseille used much lower doses of hydroxychloroquine (4 times lower on the first day of treatment).
High doses of hydroxychloroquine can therefore not only be toxic and cause serious side effects, involving the vital process, but paradoxically also adversely affect its antiviral activity. Indeed, high doses of hydroxychloroquine lower the level of interferon, a molecule essential in the fight against the virus (articles have shown the association between low levels of interferon and severe forms of the disease).
The Recovery study therefore has no interest and does not allow us to deduce anything concerning the effect of hydroxychloroquine on COVID-19. France Soir demonstrated in August 2020 that this study had most certainly killed patients.
An iniquitous fight that has been going on for months
The fight for hydroxychloroquine (HCQ) -based treatments against Covid-19 continues stronger than ever, in France and elsewhere ( Italy , United States…).
In France, this is the case at IHU Méditerranée in Marseille, at France Soir and on the Internet where many voices of doctors are expressed, individually or in groups. They have been struggling daily for months for not only to stop the ban on HCQ but also for several other curative or preventive (prophylactic) treatments such as vitamins D and colchicine, or active on early symptoms such as ivermectin and HCQ and azithromycin can finally be prescribed freely by town doctors and hospital doctors.
Most of these practitioners have been able to observe in the field the beneficial effect of HCQ administered alone or in combination with azithromycin or zinc.
To illustrate this state of affairs, there is no need to recall the last recent media attack against Professor Raoult, carried out by the intermediary of the televisual magazine “Enquête exclusive” of M6 which devoted a report to him.barely ten days ago. On this occasion, the journalist Bernard de la Villardière did not hesitate to call on a supposed Dutch medical expert. This crude charge, delivered in a soothing and mocking tone, trampled on the decency and the spirit of reason which should preside over the presentation of an eminent scientist whose only fault is to have protested against the financial powers of the laboratories that want to impose on governments, and therefore on the peoples of nations, unknown treatments, ineffective and with notorious toxic effects such as remdesivir. It was not the first time that Professor Raoult had been executed in a very disreputable manner in which we had described the springs in detail.
Thus, remdesivir, a very expensive treatment (2000 euros for 10 doses) difficult to administer (intravenously) was indecently promoted for months, by a media campaign accompanied by unprecedented brainwashing of health authorities.
We heard at Senate hearings that remdesivir, despite an uninviting therapeutic profile, had been included in the great French trial “Discovery” for: “there is no loss of chance for patients” (sic ). Recall, to add to the absurd, that this supposed antiviral treatment was given to patients in the inflammatory phase (and not viral), that is to say when the virus disappeared …
Now, a few months later, we We are talking about mRNA vaccines from Pfizer / BioNTech and Moderna, which opens a Pandora’s box.
Delayed genetic effects are completely unknown and their efficacy is estimated at 52% after the first injection in a confidence interval ranging between 29.5% and 68.4% ( BMJ of 11 Dec 2020 ), which indicates that their efficacy after the first injection. second injection may well be strongly estimated downward (<< 95%) as stated by Mediapart and other informed sources .
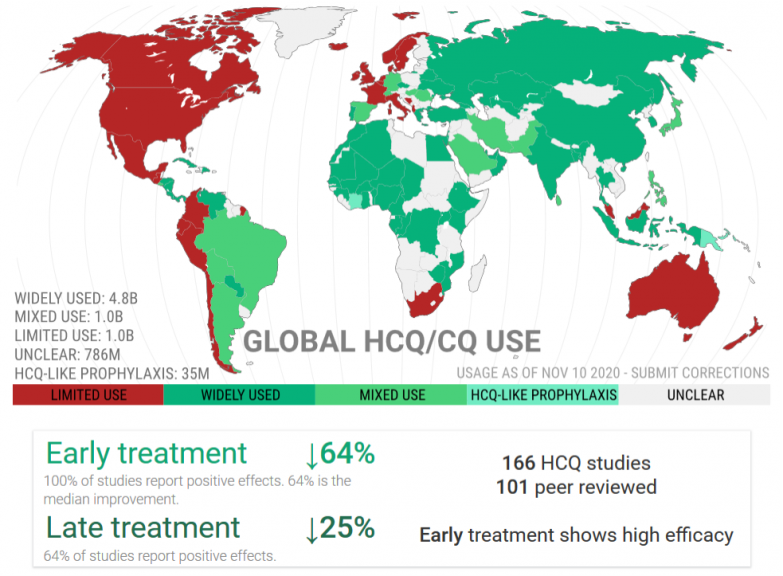
An international study has shown that 80% of scientific articles published on HCQ in 2020 were opinion pieces. Among more than a hundred real clinical studies devoted to the effectiveness of HCQ, the French press saw fit to select only a few of which one which was highlighted. This is the meta-analysis conducted by Fiolet et al., A team of young researchers, more focused on “Big Data”, having barely reached the rank of “doctor” and without in-depth experience in medical research.
Note that most of the studies use hydroxychloroquine too late when it is necessary to initiate treatment early, during the viral phase.
Importantly, one of the central elements of this meta-analysis was the result of the Recovery trial which was supposed to have demonstrated the ineffectiveness of HCQ. In the calculation of these apprentice researchers, this trial had a dominant statistical weight.
Fatal HCQ overdose demonstrated by mathematical modeling of the results of the Recovery trial
Since May 2020, France Soir, in an initial dossier followed by a series of articles ( ref1 , ref2 , ref3 , ref4 , ref5 ), has constantly argued to demonstrate that the Recovery trial was marred by an error of major conception, fraught with consequences, concerning the very dangerous overdose of HCQ.
France Soir demonstrated that the HCQ assay in this trial was developed according to an unknown protocol, ignoring pharmacokinetic data established and published for a very long time on a drug used for decades. Indeed, its dose of limiting toxicity (DLT) is established reliably around 50 mg / kg in the form of base or 65 mg / kg in the form of salt (oral route, Plaquenil), that is to say a lethal dose between 3 and 5 g in a healthy adult and around 1 g or less in children. According to Vidal, HCQ overdose begins in adults from 2.5 g.
In addition, pharmacokinetic data on HCQ had been specially established by Chinese researchers in early 2020, and published on March 9 in an international peer-reviewed scientific journal, to indicate the dosages that made it possible to obtain a plasma concentration leading to inhibition of the SARS-Cov2 virus. These dosages are debatable, because as we will see later, hydroxychloroquine has the ability to concentrate thousands of times in cellular organelles.
The recommended doses for Europeans to benefit from the treatment were a loading dose of 800 mg (in 2 divided doses of 400 mg) on the first day, followed by a maintenance dose of 400 mg (2×200 mg) on the following days.
At the IHU Mediterranean a relatively higher dose of 600 mg / day (no loading dose on D1) in 3 divided doses of 200 mg was administered in combination with azithromycin (250 mg / day over 5 days, 500 mg on D1 ) with cardiac monitoring (Lagier et al.). In this study, most of the patients (83%) were treated on an outpatient basis. The hospitalized patients considered too fragile because of their old age or cardiac history did not receive the treatment.
In L’retrospective study of AP-HP , including only hospitalized patients, the doses of HCQ were respectively 400 mg / day over 10 days (600 mg on D1), also in combination with azithromycin (250 mg / day out of 5 days, 500 mg on D1).
In the Discovery randomized trial the dose of HCQ was 400 mg / day over 10 days (2×400 mg on D1). It should be noted that a pre-print meta-analysis , carried out on 26 studies involving 44,521 patients, shows an efficacy of HCQ at low dose (<400 mg per day) on mortality, 21% reduction alone and 25% in combination. with azithromycin.
However, in the Recovery trial, conducted on hospitalized patients, 40% of whom were over 70 years of age, therefore sick and weak, the doses of HCQ were extreme. The patients received in the first 3 days of treatment the equivalent of 4 g of HCQ: 2.4 g on the first day and 800 mg per day for the following 10 days. The HCQ arm of the Recovery trial was terminated prematurely on June 5, 2020, officially due to ineffectiveness. With 23 and 25% deaths at 28 days in the standard care (SOC) and HCQ arms, it was concluded that HCQ was neither effective nor toxic.
Following which, France Soir had conducted a detailed analysis and a study by mathematical modeling of the results of Recovery from which it emerged that probably 21.4% of the 421 deaths recorded in the HCQ arm (1561 pts) probably resulted from drug poisoning associated with overdose.
In particular, an excess of 32 deaths in the first 3 days of treatment, including 15 on the first day (significant difference compared to the SOC arm, p = 0.012), could be demonstrated by the calculation. This result was corroborated by the Brazilian trial of Borba et al.which had been interrupted at the end of April, following the deaths of 39% of patients (16/41) in the high-dose chloroquine arm versus 15% in the low-dose arm (twice 450 mg on the first day, followed by 450 mg / day the following 4 days).
The authors of the Brazilian trial did not attribute the excess mortality in the high-dose arm directly to chloroquine on the pretext that the patients were all equally on azithromycin. In the trial by Borba et al. the dose of chloroquine administered was 1.2 g / day for 10 days (note that 1.2 g of chloroquine corresponds to 1.55 g of HCQ). This made Recovery the trial with the second highest dosage, in chloroquine equivalent, behind the Brazilian trial.
In fact, the Recovery doses for the first and second day combined even exceed those in the Brazilian trial with 3200 mg of HCQ versus the equivalent of 3100 mg for the Brazilian trial.
On D3, the cumulative dose was 4000 mg in Recovery against the equivalent of 4500 mg in the Brazilian trial. The publication of the failure of the Brazilian trial and the data relating to the dosage and the deaths were published by Reuters as of April 27, 2020 without apparently raising the slightest alert or investigation by the English health regulatory authorities or the committee of independent monitoring (IDMC) of the Recovery trial.

The transposition to our model of the median mortality parameters (8.4 d) and IQR-Q1 (4.5 d) published by the AP-HP had made it possible to show the significant efficacy of HCQ compared to the arm of standard care, with a mortality reduction rate of 18% identical to that measured by AP-HP in univariate restospective analysis. This result was found later (in October) to also be in agreement with the HCQ arm of the Discovery trial which gave a positive evaluation in favor of HCQ with 17% fewer deaths. Discovery was arrested without valid reason on June 30 before being able to reach the quota of patients (620) necessary for a significant statistical power. This calculated difference of 18% corresponded to a reduction total of 90 deaths, including 85 in the first 7 days, compared to the HCQ arm of Recovery!
We believe that an overdose of hydroxychloroquine in the Recovery trial potentially resulted in high toxicity within the first 3 days of treatment, although perhaps less dramatically than in the Brazilian trial, obscuring its beneficial activity.
We believe that the authors of the trial should be more transparent regarding the fact that 272 patients (349 – 77 cumulative deaths on D3) of the HCQ arm stopped treatment between D1 and D3! What are the reasons for these treatment discontinuations, which are certainly not hospital discharges? Let us also question ourselves about toxic deaths which are difficult to elucidate because overdose of HCQ can lead to acute respiratory failure just like Covid-19. A reality on which the authors of the test remain particularly discreet.
Throughout 2020, reference to overly simplistic in vitro tests led to skewed conclusions about the plasma concentrations of HCQ necessary for sufficient antiviral activity in patients’ bodies. The disastrous way whose supporting statement for the HCQ arm of Recovery was developed, under the responsibility of principal investigators Horby and Landray, testifies to this.Fooby121212
It is inconceivable that investigators responsible for a country-wide trial did not have the scientific culture and the skill to understand that the real effectiveness of a drug depends on many parameters. pharmacokinetics and physiological phenomena.
Understanding these phenomena goes beyond simplistic extrapolations from in vitro testson cell cultures. In particular, the volume of distribution of the drug between the different organs plays a crucial role as well as the way in which it interacts with the different cellular compartments of the organs involved. In addition, depending on the experimental conditions, the comparative effectiveness of in vitro studies can vary by a ratio of 1 to 25 .
To understand why HCQ can be active at doses much lower than those estimated to be reductive, it is necessary to read the scientific literature in detail. First of all, you should know that the concentration ratio between blood and plasma can vary considerably (7.2 +/- 4.2) which can mislead the levels of concentrations to be reached.
HCQ is sequestered in deep tissues and its volume of distribution (Vd) is therefore very large. This means that HCQ is found much more concentrated in the organs than in the blood plasma. For example, in animal models, HCQ and and chloroquine are concentrated in the lungs, kidneys, spleen, and liver. The lung / plasma concentration ratio varies between 27 and 177 in macaques. However, these figures are not necessarily transposable to humans with a ratio that could be lower mainly due to different metabolic processes. Indeed, CYP450 enzymes (3A, 2D6, and 2C8) catalyze the de-alkylation of HCQ to produce pharmacologically active metabolites.
Beyond this effect, hydroxychloroquine is massively concentrated in the cellular organelles that are phagosomes and lysosomes where its antiviral action is exerted. A recent article even reports a 50,000 times higher concentration between lysosomes (endosomes still attached to the membrane) than in the cytoplasm and the extracellular sector .
However, it has long been well known (1988) that HCQ / chloroquine is an inhibitor of P-glycoprotein, a protein that acts as a transporter across the plasma membrane. It allows the expulsion outside the cell of xenobiotics, ie small exogenous molecules such as cytotoxic drugs or antibiotics. We can therefore see the full therapeutic interest of treatment based on HCQ in combination with an antibiotic such as azithromycin.
In addition, we have known since 2005 that chloroquine, and by extension HCQ, shows a strong antiviral effect on the cells of primates infected with the SARS-Cov responsible for the 2002-2003 epidemic. This effect is observed when the cells are treated before and after exposure to the virus suggesting a possible prophylactic and curative effect. In addition to the well-known function of increasing basicity, this molecule interferes with the glycosylation of ACE2 preventing viral penetration of SARS-Cov, and therefore by extension that of SARS-Cov2, resulting in the inhibition of l infection of this type of virus at clinically acceptable concentrations.
Finally, HCQ and chloroquine have been shown to alkalize (strong local increase in pH) the cellular compartments involved in the phagocytosis process via lysosomes and endosomes (intracellular organelles that can encapsulate pathogens, bacilli, bacteria or viruses, during their cellular penetration), where they can be hundreds of times , or thousands of times ( up to 50,000 times ), more concentrated than elsewhere in the cytoplasm and extracellular space. These endocytotic structures can participate greatly in cellular penetration of coronaviruses, especially those containing the furin cleavage site.at the intersection of the S1 and S2 domains of the S protein. This site promotes the penetration of the virus into the endoplasmic reticulum by the endosomal route towards the Golgi apparatus enriched in furin. Furin activates the final penetration of the virus through proteolytic cleavages that take place in endosomes and lysosomes. These organelles which ensure the penetration and the transit of the virus towards the interior of the cell are the ideal place of action for HCQ. Its very high concentration would irreversibly degrade the virus at this stage. The endosomes become very strongly basic with a pH probably between 11 and 12. This is only an indicative range because the estimation of the chemical potential of protons in such an environment is subject to numerous physico-chemical factors which are difficult to assess. But in a test tube, a very alkaline medium with such a pH would allow, among other things, the deprotonation of the basic amino acids of the proteins of the viral envelope, causing their denaturation and the irreversible destabilization of the virions. It should also be remembered that the proteins of the capsid which protect the RNA of the virus are also denatured or destabilized and that the RNA itself isdestroyed by alkaline hydrolysis at pH = 12. The entire cascade of events of the viral destruction mechanism is very complex. But, for example, one can suppose that in addition to the phenomena of direct denaturation the deprotonation on the protein S of the site of cleavage of the furin which is strongly basic prevents this one from activating the virus so that it passes the membrane of the endosome. In any case, the local increase in pH can also contribute to rendering the furin itself inoperative .
However, although furin cleavage is favored in endosomes, it can also take place to a lesser extent on the cell surface, with direct penetration of an activated virus, which is why HCQ has no not absolute efficiency.
In summary, the publication of a 10-line letter in the NEJM highlights the need for French clinical research actors to reconsider all their prejudices with regard to hydroxychloroquine. These prejudices are the result of the refusal to understand that the reality of the potential benefit of a treatment and its measurement of clinical efficacy are not necessarily correlated with the results of overly reductive experiments, conducted in vitro and sometimes even only in silico ( results obtained by theoretical calculations on a computer) which can in no case reproduce the human physiological complexity.
Related: Letter to NEJM queries excessive doses of hydroxychloroquine in Covid-19 ‘Recovery’ trial
Journal of Medicine Says HCQ + Zinc Reduces COVID Deaths
Yale doctor slams Fauci for his dismissal of hydroxychloroquine in COVID treatment
Facebook Oversight Board admits Facebook was wrong to censor information about Hydroxychloroquine

