Adjunct Prof John Skerritt
Deputy Secretary
Health Products Regulation Group
John.Skerritt@health.gov.au
GPO Box 9848, Canberra, ACT
Dear Professor Skerritt,
We are writing to express our concerns regarding the role of the Therapeutics and Goods Administration (TGA) in banning Hydroxychloroquine (HCQ) for the use in Covid-19 Illness presentations and thereby criminalising its use by doctors in
this setting.
As Deputy Secretary of the TGA and the Office of Drug Control Policies, we are writing to draw your attention to various matters relating to your decision and the ramifications of these policies and directives put forth by the TGA under your leadership.
Specifically:
1: The Unwarranted Banning of an Effective Therapeutic.
We contend that the decision to specifically ban Hydroxychloroquine (HCQ) as a therapy used in combination with other agents, effectively making it unavailable for doctors to utilise to treat early Covid-19 illness, was unwarranted and did not reflect an adequate appraisal of the available medical literature regarding the safety and efficacy of HCQ.
2: Excessive and Inappropriate Sanctions and Criminal consequences for the Use of HCQ in the treatment of Early Covid Illness.
The risk of serious sanctions or criminal convictions for doctors now legislated for in various legislatures for the use of HCQ in the setting of early covid illness are the result of decisions made by the TGA under your leadership, based on recommendations of the NATIONAL COVID-19 CLINICAL EVIDENCE TASKFORCE (NC19T).
This is a serious encroachment on the rights of citizens and an unnecessary interference in the doctor-patient relationship by governments. The result of these excessive provisions has resulted in undue consequences on many patients as well as doctors.
3: A failure to review the available evidence regarding the effectiveness and safety of Hydroxychloroquine for use in Early Covid Illness.
We contend that the decision to ban Hydroxychloroquine, and thereby deny Australians access to well-evidenced and effective treatments for early covid illness, were based on an inadequate review of the medical literature and an inaccurate and erroneous reading of the available evidence.
The recommendations made by the NC19T, under the leadership of Associate Professor Julian Elliot, appear to have been taken on by the TGA without due consideration of the available international evidence and without an appropriate and timely review process, especially in the context of the rapidly growing body of available evidence which successfully challenges and contradicts the NC19T recommendations.
4: Accountability and the Negative Consequences of Banning
Hydroxychloroquine:
We contend, based on international evidence and experience, that the consequences of the decisions by the TGA to ban HCQ have had serious negative consequences on the health and well-being of many Australian citizens and may have directly contributed to the deaths of hundreds of patients.
With such serious health consequences, that have also resulted in other negative impacts in our community, including significant economic and social costs, we believe an independent inquiry should be recommended to analyse the timing and nature of the decision making, with a focus on the responsibility of these decisions.
We believe medical doctors, give the great responsibility they are given, should always be held accountable for their actions and decision making especially when the consequences of such responsibility results in considerable harms.
We contend that the decision-making process in relation to the banning of HCQ should be considered in the same way.
In short, we contend that Australian citizens should not be denied access to safe, effective and well-evidenced therapies for early Covid-19 illness.
We strongly advise that the banning of HCQ by the TGA be urgently reviewed and revised based on the large body of medical and scientific evidence that is now available.
Challenging the TGA’s Four Reasons for Banning HCQ:
We respectfully offer the following information as a background and overview of the current state of scientific knowledge related to HCQ and its use as a part of early treatments of Covid-19 illness, to assist the TGA review and reconsider its decision to ban HCQ for Covid-19 illness.
A: Lack of Efficacy of HCQ in Treating Covid-19 Illness:
The TGA seems to have relied on Australia’s NC19T to conclude that HCQ lacks efficacy in the treatment Covid-19 illness.
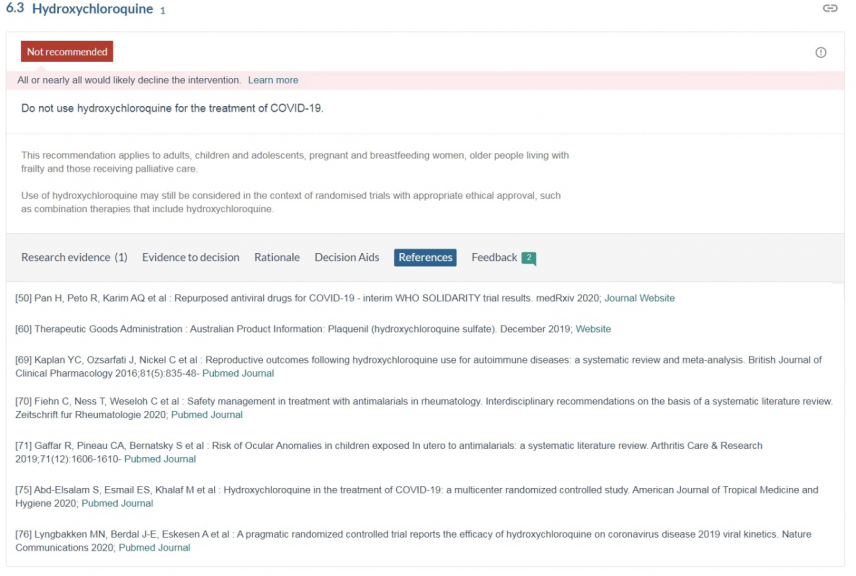
We contend that the recommendation by the NC19T: “Do not use hydroxychloroquine for the treatment of Covid-19”, relies on studies with flawed methodology and is the result of an inadequate review of the available literature and the current state of scientific knowledge.
Every study relied upon by the NC19T as listed in their references was deficient in one or more of the following four ways:
i. HCQ was NOT Assessed for Phase 1 (early stages) of Covid-19 Illness.
Studies that utilise HCQ Protocols only in hospitalised patients or in the late phase of the illness, are fundamentally flawed as they do not address the efficacy of HCQ in combination therapy for Early/Phase 1 of Covid-19 illness.
The timing of treatment with HCQ is critical and is consistent with the known scientific basis of the mechanism of action of the drug and the nature of the phases of the illness. It is now well accepted that outpatient treatment using HCQ must occur in the first few days of infection and development of symptoms in order to have significant efficacy.
ii. Absence of Zinc in the HCQ Protocols:
Studies of HCQ protocols which do not include Zinc as part of the
combination therapy. The inclusion of zinc is critical for the efficacy of
HCQ in the early phases of Covid-19 illness. HCQ acts as the ionophore for Zn to enter the cell membrane and block Virus Replication – see: http://tribeqr.com/v/hcqthescience
iii. Use of Excessive or Toxic Doses of HCQ:
Studies that use dangerously high doses of HCQ, well above t
recommended TGA guidelines for HCQ usage for any recognised
condition, are fundamentally flawed as evidence for examining the safety profile of HCQ in the setting of Covid-19 illness.
iv. Absence of Patient Risk Stratification in Study Design:
Studies of HCQ Protocols which lack detailed patient risk stratification, blur or ‘dilute the efficacy of HCQ combination therapy by including young and low-risk patients who would likely recover regardless of any therapeutic intervention. Studies of HCQ protocols without appropriate risk stratification of the study population lack the rigour to assess the efficacy of HCQ, which is considered to be particularly efficacious for patients aged over 50 with comorbidities and higher risk stratification.
[A detailed analysis and critique of each of the studies that were relied upon by the NC19T’s to formulate its recommendation: “Do not use hydroxychloroquine for the treatment of Covid-19”, can be provided on request.]
B: Poor Safety Profile of HCQ:
The TGA states: “Clinical trials are underway around the world examining their potential to treat COVID-19. However, these medicines pose well-known serious risks to patients including
cardiac toxicity (potentially leading to sudden heart attacks), irreversible eye damage and severe depletion of blood sugar(potentially leading to coma).”
However, the available evidence now establishes the safety of HCQ inappropriate doses in the setting of treating of Early/Phase 1 Covid-19 Illness. HCQ is sold over the counter without a prescription in many countries where malaria is prevalent. Its safety profile has been well-established with over 50 years of use in many different countries.
The claims about cardiac toxicity, especially an increased frequency of ventricular arrythmias when HCQ is used to treat Covid-19, have now been refuted and retracted. The influential Lancet article which first raised these concerns was published on May 22nd 2020 was retracted on June 5th 2020 due to concerns about the quality and veracity of the data.
In its retraction article, the Lancet editors explained the reason for its retraction: “several concerns were raised with respect
to the veracity of the data and analyses conducted by Surgisphere Corporation and its founder and our co-author, Sapan Desai”.
It also seems that the TGA’s assessment of HCQ’s safety profile may have also been based on the now-discredited Recovery Trial in the UK, in which very sick patients were overdosed with HCQ, receiving doses of 2400mg. It is now understood that the ‘error’ in dosage resulted from a mistaken substitution of appropriate HCQ doses with Hydroxyquinoline doses.
Conclusion: HCQ has a well-established and proven safety profile for use inMalaria and inflammatory disorders such as Rheumatoid Arthritis and Systemic Lupus for over 50 years. Its safety profile in the early treatment of Covid-19 illness has also now been well established.
C: Potential Shortage Issues:
The TGA initially banned HCQ via the “Poisons Standard Amendment (Hydroxychloroquine and Salbutamol) Instrument 2020”, which amends the Poisons Standard February 2020 in relation to the substances HCQ and Salbutamol. The reasoning for the ban was provided via your release, “New Restrictions on Prescribing Hydroxychloroquine for COVID-19”, which was
released on the 24th March 2020.
In that document, it states: “Recent reports of increased off-label prescribing of medicines containing Hydroxychloroquine have raised concerns that this will create a potential shortage of this product in Australia.”
https://www.tga.gov.au/alert/new-restrictions-prescribing-hydroxychloroquinecovid-19?fbclid=IwAR3M9pRUB-Ha8-3cMzGLO08ZXXyUOwQdNmEXa6-
EbQQpxg44RLgqtiyezwM
However, the reasoning appears to be flawed and outdated as it was public knowledge that in April 2020, Clive Palmer had donated 32 million doses of HCQ to the Australian people.
On the 8th May 2020, one month after Mr Palmer’s donation of HCQ, the TGA released an amendment, reaffirming the TGA’s reasoning regarding the issue of HCQ supply shortage, stating:
“To reflect Therapeutic Goods Administration (TGA) regulatory changes regarding Hydroxychloroquine (described below), the Department of Health adjusted the Pharmaceutical Benefits Scheme (PBS) listing (link is external) for Hydroxychloroquine from 1 May 2020. This change will help minimise the risk of PBS prescriptions being supplied to patients accessing Hydroxychloroquine for unapproved uses and ensure that patients who rely upon on this medication for approved uses will have continued access.”
https://www.tga.gov.au/alert/amendments-new-restrictions-prescribinghydroxychloroquine-covid-19
Conclusion: There is currently no shortage of HCQ doses for Australian citizens.
Any possible future HCQ shortages should be managed by government efforts to access supplies from international sources. Therefore, the TGA should not be advising the Australian government to deny at-risk Covid-19 infected patients the potential life-saving benefits of a safe, well established and inexpensive treatment protocol, on this basis.
Recommendations and Recommended Resources
We recommend that the TGA instigates its own independent review of the safety and efficacy of HCQ in combination therapies for the early treatment of Covid-19 illness, or support an independent inquiry for this purpose.
We recommend the TGA involve both national and international expertise, especially taking into account the experience of many other countries where HCQ has been extensively used as a prophylactic and as a treatment of early Covid-19 illness. Such countries include: India, Russia, Turkey, Greece, Ukraine, Morocco and Indonesia as well as some areas of the United States of America.
Some of these countries’ Governments actively promote the use of HCQ as prophylaxis or treatment within the first 3 days of symptoms of Covid-19 illness, in combination with other agents. All have shown this approach to be efficacious and safe with a significant reduction in hospital admissions and mortality rates.
A 70% reduction in mortality has been demonstrated in a comparative study between countries allowed access to HCQ and countries that have denied HCQ access to their citizens. This extraordinary finding can be accessed at the following website: https://hcqtrial.com/
There is now a large and growing body of medical evidence, expertise and knowledge base to support the safe use of HCQ in the setting of Covid-19 illness.
At our request several leading international experts have offered to assist the TGA, or any other Australian healthcare institutions or bodies, as expert advisors and/or expert witnesses. Their details and several resources including various recommended websites are attached in Appendix 1 and 2 below.
In summary, we contend:
- The current evidence now clearly shows that HCQ, in combination with other medicines, provides a very safe and effective therapeutical regime for the treatment of SARS-like diseases, especially in its early stages.
- There is no current shortage of HCQ; there are 32 million extra donated doses available to the Australian Government. Limiting its current use and availability use of HCQ risks further increases in mortality and hospitalization for at-risk patients into the new year. We consider such a course of action to be a breach of good medical practice and ethics.
- We believe the TGA, by banning a safe and effective medication that has been available for off label prescription for decades, to have inappropriately interfered in the doctor-patient relationship, thereby exceeding its authority in terms of its rights and responsibilities, and has put at risk the health and safety of many Australians.
- There is an urgent need to review the TGA’s decision-making in this regard. We are calling for the TGA to support an independent public inquiry into the decision to ban HCQ as a therapy for Covid-19 illness and to provide a transparent account of those responsible for the decision and the evidence that was used and not used to sustain their decision.
- In the interim, we contend that there is an urgent need to revoke the decision to ban HCQ for use in early Covid-19 illness.
In conclusion, we respectfully request that you, or other representatives of the TGA, respond to the following questions:
- To the best of your knowledge, what was the scientific and medical
knowledge that was used to inform the TGA’s decision to declare HCQ as unsafe for human consumption in the setting of Covid-19 illness? - Can you provide the exact literature that was relied upon to form and sustain the view that HCQ was ineffective and/or dangerous, and thereby required being withdrawn from use to prevent medical doctors treating their patients with Covid-19 illness?
- To the best of your knowledge, did the TGA rely solely upon the
recommendations of Australia’s National Covid-19 Taskforce, headed byProfessor Julian Elliot, to support its decision? - To the best of your knowledge, did the TGA conduct its own research or inquiries into the efficacy and safety of HCQ prior to its initial decision to ban HCQ for use in Covid-19 illness?
- To the best of your knowledge, did the TGA put in place an ongoing review of the available scientific and medical evidence, to sustain or change its stance on the banning of HCQ? If not, did the TGA instruct the NC19T to do so?
- To the best of your knowledge, can you explain the initial decision-making processes (and processes of review if they existed) to inform the TGA? In particular, what persons and agencies or organisations that were specifically involved in the process?
- To the best of your knowledge, can you explain the communication
protocols and processes between the TGA and Government as well as Statutory bodies and Chief Health Officers (CHOs) and Chief Medical Officers (CMOs), that led to legislative sanctioning and criminalisation of medical doctors for using HCQ to treat Covid-19 patients? - To the best of your knowledge, what person or persons do you consider to be ultimately responsible for the decision to ban the use of HCQ to treat early Covid-19 illness?
- If it can be shown that the direct and consequential interference by
Government and its advisors ( including the TGA, NC19T and the various CHOs and CMOs) to ban HCQ resulted in and contributed to identifiable harms and deaths, do you consider it the responsibility of Government, the TGA or the advisors upon whom the TGA relied for its decision, to be accountable for such outcomes? - In particular, should the consequences of the decision to ban HCQ be the sole responsibility of the TGA and its officers or should that responsibility be shared by other parties including Health Ministers, Chief Health Officers and the leadership of the Covid-19 National Taskforce?
We look forward to your responses at your earliest convenience.
Yours Sincerely
Dr. Eamonn Mathieson
Secretary, Covid-19 Medical Network
admin@covidmedicalnetwork.com
Mobile: 0412 664 232
Covid-19 Medical Network Ltd
ABN: 74 645 786 401
admin@covidmedicalnetwork.com
PO Box 2607 Camberwell West 3124
www.covidmedicalnetwork.com
www.cmnnews.org
Appendix 1:
The following websites may serve as an excellent resource and concise summary of the current state of scientific knowledge regarding HCQ and early treatments and prophylaxis of Covid-19 illness.
• c19study.com
• metahcq.com
• hcqtrial.com
• covexit.com
• aapsonline.org
• americasfrontlinedoctors.com
Appendix 2:
The Australian CMN group is in contact with the following international experts, all of whom have considerable practical experience in treating Covid-19 patients.
They have all read and endorsed this letter.
These experts have offered their services as expert witness and as resources of information for the TGA . They can be contacted via their personal email addresses as detailed below.
Alternatively, representatives of the CMN can assist and private online conferences with any of these experts, with or without the attendance of the TGA’s current local advisors.
• Professor Peter A. McCullough, M.D., M.P.H.
Vice Chief of Internal Medicine, Baylor University Medical Centre
peteramccullough@gmail.com
www.aapsonline.com
Prof McCullough’s peer-reviewed protocol on the early treatments Covid-19 was published in the American Medical Journal on August 7th 2020. Entitled:
Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2(COVID-19) Infection, the article can be viewed here.
His booklet on ‘Early Outpatient Treatments of Covid-19’ can also be found here:
https://aapsonline.org/covidpatientguide/
• Professor Harvey Risch, M.D., PH.D.
Professor of Epidemiology, Yale University
harvey.risch@yale.edu
[Prof Risch and Prof McCullough were lead witnesses at a recent US Homeland Security Senate Hearing on November 19th, 2020, entitled: Early Outpatient Treatment: An Essential Part of a Covid-19 Solution. A 27-minute version of the hearing can viewed here:
https://www.youtube.com/watch?v=ftq6lmRlKgQ&feature=youtu.be]
A transcript of the Hearing can be accessed here:
https://www.c-span.org/video/?478159-1/senate-hearing-covid-19-outpatienttreatment&live
• Professor Dolores Cahill:
Professor of Medicine, University College, Dublin, Ireland.
DoloresCahill@gmail.com
• Dr Vladimir Zelenko MD:
zz613@hotmail.com
Dr Zelenko’s study, the first to include risk stratification, can be found at the following link:
https://www.sciencedirect.com/science/article/pii/S0924857920304258
• Dr Robin Armstrong MD
robarmstr@hotmail.com
• Dr George Fareed MD
gfareed@gmail.com
Appendix 3.
Studies relied on by the National Covid-19 Taskforce to recommend: “Do not use Hydroxychloroquine for the treatment of COVID-19.” (References cited November 18th, 2020)
Related: ‘Only a one in 17 billion chance hydroxychloroquine doesn’t work’: medical professor

